Recent developments of liquid-phase microextraction techniques directly combined with ESI- and MALDI-mass spectrometric techniques for organic and biomolecule assays
Suresh Kumar Kailasa
a,
Vaibhavkumar N. Mehta
a and
Hui-Fen Wu
*bc
aDepartment of Applied Chemistry, S. V. National Institute of Technology, Surat – 395007, India
bDepartment of Chemistry and Center for Nanoscience and Nanotechnology, Institute of Medical Science and Technology, Doctoral Degree Program in Marine Biotechnology, National Sun Yat-Sen University, Kaohsiung, 804, Taiwan. E-mail: hwu@faculty.nsysu.edu.tw; Fax: +886-7-5253908; Tel: +886-7-5252000-3955
cSchool of Pharmacy, College of Pharmacy, Kaohsiung Medical University, Kaohsiung, 806, Taiwan
First published on 6th February 2014
Abstract
The development of rapid, simple and reduced solvent consumption techniques for sample preparation is important for the isolation and preconcentration of organic and biomolecules from complex matrices. Miniaturized solvent-based extraction techniques have been intensively applied as sample pretreatment tools for the preconcentration of biomolecules from biological samples prior to their identification by matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS). This review provides an overview of miniaturized solvent extraction methods and their efficient extraction of trace or ultra-trace analytes and their identification by MALDI-MS. We discuss the recent developments of miniaturized liquid-phase extraction approaches, such as liquid-phase microextraction (LPME) and single-drop microextraction (SDME) techniques, for organic and biomolecule pretreatment prior to MALDI-MS analysis. We also provide an update on the state-of-the-art and promising prospects of LPME techniques directly combined with ESI- and MALDI-MS techniques for the analysis of trace levels of organic and biomolecules in complex matrices. We also outline the advances of using liquid microjunction surface sampling probes coupled with MS for trace level analyte assays. Nanoparticle-assisted microextraction coupled with MALDI-MS for organic and biomolecule analysis, and the future trends of potential LPME applications are also highlighted.
1 Introduction
Over the last several decades, mass spectrometry (MS) has emerged as an important analytical technique for the analysis of a wide variety of species in various samples. It is an essential, robust, sensitive, and reliable tool for detecting target species at low concentrations (femto-mole levels) in micro-litre sample volumes, which are not easily detected by other conventional techniques.1 To date, many ionization methods of mass spectrometric tools have been developed for qualitative and quantitative analysis of various analytes. Since their inception in 1980s, the two powerful soft ionization methods of electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI) have been a popular choice for organic, inorganic and biomolecule analysis.2–4 However, MALDI-MS has became a major analytical tool for organic and biomolecule analysis from environmental and biological samples because it can offer high sensitivity, rapid results, and a wide mass range (>100 kDa).2–6 Despite the significant advances made in instrumental design over recent years, sample pretreatment procedures are required prior to MS analysis, especially when the target species are at a trace level concentration with significant interferences.The sample preparation process is a vital step in any instrumental analysis, which involves the isolation/preconcentration of target analytes from a sample matrix, as they are typically at extremely low concentrations such as ultra-trace levels. The analyses of organic and biomolecules in complex samples are important due to the need to understand more about their amounts in sample compositions and their effects on biological pathways.7–9 Even though there has been intensive advances made towards the development of analytical instrumentation for determining analytes in various samples (biological and environmental), most of the analytical instruments require tedious sample preparation procedures in order to extract, and preconcentrate the analytes from the sample matrices. Most of the instruments cannot directly detect the trace level analytes that are present in sample matrices due to sample complexity.7 Therefore, the analysis of organic and biomolecules from environmental, biological and industrial samples generally requires appropriate sample pretreatment and extensive separation prior to instrumental analysis. However, as the sample matrices may contain acids, bases, proteins, salts and other organic compounds that may have similar chemical properties to the analyte of interest,8,9 sample preparation steps become a crucial part of the analysis. The development of analytical methods involves several steps including sampling, sample preparation, separation, analysis, and data evaluation with computational statistics. These steps have significant impacts on the analytical efficiency of the method (accuracy, precision and sensitivity).7–9 This is due to the interferences of other species in the sample matrices, which can lead to a reduction of the instrumental and analyte signals in the spectra. Hence, sample preparation helps to eliminate the unwanted species from sample matrices with reduced solvent and sample volumes, which facilitates to the preconcentration of analytes. The development of green analytical methods is a very important research area, along with the detection and quantification of target species in various matrices such as environmental and biological samples.7–10
The sample preparation (extraction, separation, and preconcentration) is a basic step for the analysis of target analytes by various analytical instruments. The choice of the applied sample treatment technique depends on the nature of the biomolecules, the matrix and the sample volume. In this sense, several extraction techniques can be used to extract the target analytes from the sample matrices prior to analytical instrumentation (Fig. 1). Among these techniques, the most traditional and classical technique is liquid–liquid extraction (LLE),11 which remains the most frequently used technique for isolation, separation and analytical chemistry. It is one of the most popular conventional separation techniques that depends on the analyte solubility in different liquid phases; usually between an aqueous and an organic phase. However, this technique has some drawbacks such as requiring large amounts of organic solvents that are toxic and expensive, time-consuming, environmentally unfriendly, laborious, tedious, and difficult to automate. In order to improve the classical liquid extraction techniques, miniaturized extraction techniques have been proposed for sample treatment in environmental and biological samples prior to MS analysis. The solvent microextraction (SME) technique deals with the extraction and preconcentration of a target species from sample matrices by using solvent volumes from 0.5 μL to 100 μL.7–12 It is well suited to the extraction, purification and concentration of volatile, non-volatile, polar, non-polar, ionic and metallic analytes from environmental and biological samples.13–18 In recent years, much effort has been devoted towards the development of green analytical methods for the rapid preconcentration of analytes from environmental and biological samples by using minimum amounts of solvent and reagents and a drastic reduction of laboratory wastes.8–20 Since 1995, many LPME techniques have been coupled with various analytical instruments including mass spectrometry for the identification of organic, inorganic and biomolecules in various samples.7–28 Fig. 2 shows the classification of LPME based on their extraction modes. These miniaturized solvent extraction tools have received increasing attention in different fields and have been popularly applied in modern analytical studies for the isolation/concentration of trace level targets with minimized solvent and sample volumes.7–25 The aim of this review is to introduce the recent advances made in miniaturized solvent extraction techniques (SDME and LLME) directly coupled with ESI- and MALDI-mass spectrometric tools for the analysis of organic and biomolecules. The LPME parameters such as organic solvent, pH, extraction time, stirring rate and addition of salt in miniaturized solvent microextraction techniques for the preconcentration of organic and biomolecules prior to MALDI-MS techniques are also discussed. Further, we also discuss liquid microjunction surface sampling probes coupled with MS for organic and biomolecule assays. Importantly, we also review the remarkable progress and developments made on the integration of nanomaterials with solvent microextraction techniques prior to their identification by MALDI-MS. These discussions will be limited to the analysis of organic and biomolecules by SME techniques directly coupled with MALDI-MS techniques.
2 Parameters effects on the efficiency of miniaturized solvent extraction techniques
Miniaturized solvent extraction techniques were first introduced by the Dasgupta and Cantwell groups in 1996.26,27 Since then, several research groups have introduced solvent microextraction techniques with slight modifications or major variations in modes of microextraction design. To avoid confusion with SME terminology, Kokosa reviewed recent innovations of SME for target species preconcentration and classified the techniques into two broad categories, i.e., one is solvent exposed microextraction and second one is membrane-protected solvent microextraction.16 According to the mode of SME operation, single-drop microextraction (SDME), headspace single-drop microextraction (HS-SDME), liquid–liquid microextraction (LLME, also referred to as directly-suspended droplet microextraction, DSDME), liquid–liquid–liquid microextraction (LLLME) and dispersive liquid–liquid microextraction (DLLME) techniques will come under solvent-exposed microextraction techniques. Furthermore, the membrane-protected solvent microextraction are classified into two categories, such as hollow-fiber-protected 2-phase microextraction (HF(2)-ME) and hollow-fiber-protected 3-phase microextraction (HF(3)ME). It was noticed that the some experimental parameters (solvent, pH, extraction time, stirring rate and addition of salt) influence the extraction and preconcentration of target species in both SME techniques (SDME and LLME) prior to their identification by various analytical instruments.7–25 The important theoretical aspects, recovery and enrichment factor calculations of SME techniques are well documented in the literature.20–24,28,29 Various parameters such as organic solvent, exposure time, agitation, organic drop volume, drop stability and salt concentration can influence the extraction efficiency of the SME system. In this section, we briefly discuss the effects of experimental parameters such as solvent, pH, extraction time, stirring rate and addition of salt on the isolation and preconcentration of target species from environmental and biological matrices prior to MS analysis.2.1 Nature of the analytes
The SME is based on liquid phase separations. For example, the two-phase SDME technique involves the extraction from an aqueous phase to an organic phase. Similarly, the three-phase SDME technique involves the extraction of an analyte from an aqueous phase to an organic phase, followed by a transfer from the organic phase to an aqueous phase.11 The extraction/preconcentration efficiency of the SME technique is not only dependent on the nature and volatility of the analytes, but also dependent on the SME configurations. The best extraction/preconcentration of non-polar or moderately polar, volatile and semi-volatile analytes can be achieved by headspace SDME. Further, direct immersion SDME is well suited to the extraction/preconcentration of non-polar or moderately polar higher molecular weight, and semi-volatile compounds from environmental and biological matrices. Based on the polar nature, the SME technique needs to be derivatized to ensure recovery for high polar compounds, especially when the sample is a liquid. With recent advances in SME techniques, most of the analytes such as volatile compounds, phenols (alkyl, chloro, nitro), organic acids, amines, long chain fatty acids, alkylphosphonic acids, dyes, pesticides, polymers, explosives and drugs are extracted/preconcentrated by using miniaturized solvent extraction tools. The enrichment factor can be calculated from the ratios of the equilibrium concentrations of the analytes in two phases.2.2 Effect of solvents and drop volume
It is well known that the SME technique is based on the phase separation phenomenon that a solute or analyte distributes itself in a certain ratio between two immiscible solvent phases, such as an aqueous phase (water) and organic phase (organic solvent). On the other hand, the analytes are extracted from a sample solution using a miscible or immiscible organic solvent in a microliter volume. In some cases, water-miscible organic solvents such as acetone, 1,4-dioxane, acetonitrile, ethyl acetate and tetrahydrofuran are also used as solvents to induce phase separation.14 A wide variety of physico-chemical property solvents (water-miscible solvents, water-immiscible solvents, ionic liquids and supercritical fluids) are used as solvents in SME prior to instrumental analysis. However, our main focus is on the solvents used in SME, which are directly coupled with MALDI-MS. Since, the selection of an organic solvent plays a key role in the enhancement of the extraction efficiency and the target species signal intensities. An ideal organic solvent should have the following characteristics; (i) high affinity towards target analytes from sample matrices, (ii) water insolubilty to prevent dissolution into the aqueous phase, (iii) low volatility to prevent the evaporation of solvent during extraction, (iv) chemically inertness and stability during extraction and (v) a moderate viscosity to cling onto the tip of a syringe needle for SDME and also the density of the organic solvent in SDME should be equal to or greater than the density of water otherwise the drop covers the syringe.30 To achieve efficient analyte extraction and enrichment, various solvents such as tetrachloroethane, dichloromethane and acetone, 1-octanol, decane, 1-butanol, iso-octane, chlorobenzene, di-n-ethylether, n-hexane, dichlorocarbene, tetrachloride carbon, o-xylene and toluene have been used as solvents for the SME of organic and biomolecules prior to their identification by MALDI-MS (Table 1).| Analyte | Experimental conditions | Linear range | LOD/LOQ | MS technique | Sample | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Solvent | Volume (μL) | Extracting time (min) | pH | Stirring rate (rpm) | ||||||
| a ng mL−1.b μM. | ||||||||||
| Monensin | CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene (1 toluene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
1.5 | 10 | — | 240 | 10–100a | 6.7–12.4a | AP-MALDI-MS | Soil, surface water, and human urine | 37 |
| Quinine | m-Xylene | 4.0 | ∼7.7 s | 10.0 | — | 0.31–21.5b | 0.18 and 0.24b | AP-MALDI-MS | Water, urine, plasma | 38 |
| AM, NTP, QN, IP, TMP | Xylene | 1.0 | 10 | — | 240 | — | 0.3–1.6b | AP-MALDI-MS | Urine | 39 |
| CPC, MTOAC, CTAB and TOAB | Octanol | 0.8 | 7.0 | — | 240 | 50–1500a | 10a | AP-MALDI-MS | River water and municipal wastewater | 40 |
| Dopamine | Octanol | 2.0 | 5.0 | 8.0 | 240 | — | 25–40 ppm | AP-MALDI-MS | Urine | 41 |
| MA | Hexane | 4.0 | 60 | 5% AcOH | 1100 | 10–1000a | 51 ± 4, 127 ± 9a | SDME-DESI-MS | Water | 42 |
| IAA, IBA, JA, SA, ABA, GA3 | Anisole and ethyl acetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
1.0 | 15 | 1.1 | 9500 | 2–25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000a 000a |
0.65–72a | SD-LLLME-DART-MS | Pineapple, pear, and watermelon | 43 |
| Small molecular aldehydes | MeOH + diphenylamine + 2,5-DHB | 2.0 | 30 s | — | — | 100–1700a | 0.003–0.0103a | MALDI-FTICR-MS | Single puff smoke | 44 |
| Valinomycin and gramicidin D | Octanol | 2.0 | 5 | — | 240 | 0.5–7.0b | 0.073–0.300b | AP-MALDI-MS | Water, urine, plasma | 45 |
Besides organic solvents, room temperature ionic liquids (ILs) are considered as environmentally friendly solvents which can serve as alternative solvents because of their unique physicochemical properties (negligible vapour pressure, good thermal stability, tunable viscosity and miscible/immiscible properties in water and organic solvents), which depend on the nature and size of their cationic and anionic constituents. Recently, several microextraction techniques were performed using various ionic liquids as solvents for the extraction of metals, metalloids, biomolecules, and organometals prior to their detection with analytical instruments.
Furthermore, the sample volumes of donor and acceptor solutions have a strong influence on the SME efficiency. The volume of the sample should be selected to provide sufficient sensitivity for the assay, but this has to be optimized since the extraction time increases with increasing sample volume. The SME extraction of target species in biofluids are usually between 0.1 and 4.0 mL, however, the volume of the acceptor solution may depend on the microextraction mode and the analytical technique. The following volumes of the acceptor solution are usually used in SME coupled with mass spectrometric techniques; 0.5–2.0 μL for SDME; 10–300 μL for LLME.
| Analyte | Experimental conditions | Linear range | LOD | MS technique | Sample | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| Solvent | Volume (μL) | Extracting time (min) | pH | ||||||
| a nM.b ng mL−1.c ng g−1. | |||||||||
| Volatile basic components | TFA + H2O + acetone + 2,5-DHB | 3.0 | 15 | >8 | — | — | MALDI-FTMS | Tobacco | 47 |
| Nicotine | TFA + H2O + acetone + 2,5-DHB + glycerol | 3.0 | 4 | — | 1–10a | 0.12a | MALDI-FTMS | Cigarette smoke | 48 |
| Lipopoly-saccharides | Modified enzyme–phenol–water | — | — | — | — | — | TLC-MALDI-MS | E. coli lipid A and Bordetella lipopolysaccharides | 49 |
| Basic drugs | 1-Octanol + 10 mM HCl and toluene | 10.0 | 30 | — | 50–2000b | 25–100b | DESI-MS | Urine | 50 |
| ACE, TMX, THIA, CLO, IMI | ACN + HCOOH + CHCl3 | 100 | >2.0 | — | 0.1–7500c | 0.02–1.0c | APCI-IT-MS/MS | Honey | 52 |
| NTP | Toluene | 3.0 | 5.0 | 7.0 | 0.019–1.9a | 6.26–94.9a | AP-MALDI-MS | Urine and plasma | 53 |
| Aplysia californica B1 and B2 motor neurons | 2,5-DHB + acetone + water | 0.5 | — | — | — | — | MALDI-MS | Single cell | 54 |
| Angiotensin I and digested proteins | CHCA + 3-AQ + Q | 2.0 | 1.0 | 4.0–10.0 | — | 1.25a | MALDI-MS | Peptides and protein digests | 55 |
2.3 Effect of pH
The pH of the solution plays a key role in the dissociation equilibria between the acceptor and donor phase, affecting the solubility of acidic and basic analytes, which allows to enhance extraction efficiency of the system. Therefore, the extraction efficiency is improved by adjusting pH of the donor/acceptor phases; noticeably analytes are greatly preconcentrated by adjusting the pH of the donor phase. In all SME techniques, the sample pH adjustments are essential for the extraction of acidic and basic analytes.2.4 Sample agitation and extraction time
Sample agitation enhances the distribution rates (extraction kinetics) of the analytes from one phase to another phase, which leads to an enhanced extraction efficiency with a reduced extraction time. Currently, the following mechanical tools are used for sample agitation during SME; stirring, vibration and vortexing. The sample was stirred using a magnetic stir bar at different rpm ranging from 150–1000 rpm, based on the analyte interest in SDME. It was observed that higher stirring rates can lead to bubble formation, solvent evaporation and instability of the drop at the needle edge.29 Generally, the vibration and vortex stirring are used and the sample vials are stirred ranging from 500–1000 rpm for LMPE. Therefore, sample agitation allows the continuous exposure of the extraction surface to the aqueous sample, which facilitates the enhancement of the extraction kinetics and extraction efficiency.Consequentially, equilibrium times may differ from application to application depending on parameters such as the geometrical configuration of the equipment, the distribution constant, and the level of sample agitation. Most of the SME studies required 1–60 minutes to reach equilibrium.
2.5 Effect of salt addition
The extraction efficiency is also enhanced by adding salt to the SME system, especially for moderately polar and low molecular weight volatile compounds. The SME efficiency is increased due to the following reasons; (i) salt addition enhances the ionic strength of the solution which leads to an improved extraction efficiency; (ii) the signal intensities of the target analytes were greatly enhanced in the mass spectrum due to the formation of sodium/potassium adduct ions. (iii) The addition of salt leads to the reduction of water molecules which are attached to the target species. However, the extraction efficiency can be increased with a certain concentration of salt, after that it leads to a decrease in the diffusion rates of the analytes from the aqueous phase to organic phase.33 Furthermore, the solubility of the extracting solvent is decreased with increasing ionic strength, at a saturated salt concentration, the drop can be dislodged because of the undissolved particles in the droplets. Several reports have described the effect of salt addition in SME, with that the extraction efficiency of the analytes being monitored by observing the signal intensities in the mass spectrum. The signal intensities of the target species were increased by the addition of salt (NaCl) from 1 to 3%, after that no further changes were observed.33 Therefore, addition of salt in SME acts as an adsorber to preconcentrate and to enhance the interactions of the target species with the solvent system.34,35 However, in some investigations, the salt addition does not have an effective influence on the extraction efficiency.283 Solvent microextraction techniques directly coupled with MALDI-MS
Theoretically and practically, SME techniques (SDME and LLME) are very simple and have the ability to extract and to preconcentrate any type of analyte in a minimal volume of liquid sample. Theory, and analytical applications of SME (SDME and LLME) techniques coupled with various analytical instruments including UV-visible, fluorescence, atomic absorption spectrometry, inductively coupled plasma mass spectrometry, liquid chromatography- and gas chromatography-mass spectrometric techniques have been reported.8–32,36 To date, 17 review articles have been presented on solvent microextraction techniques coupled with various analytical instruments, except SME techniques directly coupled with MALDI-MS. With this large available database, the present section deals with the SME (SDME and LLME) techniques directly coupled with MALDI-MS for analysis of organic and biomolecules in various sample matrices. Apart from that, we will give an overview on nanoparticle-integrated miniaturized solvent extraction techniques coupled with MALDI-MS for the analysis of organic and biomolecules.| Nanomaterials | Size (nm) | Extracting solvent | Volume (μL) | Optimal conditions (extraction time; sample agitation and pH) | LOD | Analytes | Technique | Reference |
|---|---|---|---|---|---|---|---|---|
| a μM.b fM.c nM.d cfu mL−1. | ||||||||
| TAAB-Au NPs | <10 | Toluene | 0.8 | 1 min; 240 and 6.0–11.0 | 0.2, 0.17a | Met-enk, Leu-enk | SDME-AP-MALDI-MS | 81 |
| MPIMP-Au NPs | — | Toluene | 1.0 | 30 min; 200 and 6.0 | 13.2–53.1b | Peptides, proteins and milk proteins | SDME-MALDI-MS | 82 |
| TOAB-Ag NPs | <50 | Toluene | 0.8 | 2 min; 240 and 7.0 | 160, 210c | Met-enk, Leu-enk | SDME-AP-MALDI-MS | 83 |
| BMIHFP – Pt NPs | 10 | Ionic liquid | 1.0 | 5 min; 350 | 106d | E. coli and S. marcescens | SDME-MALDI-MS | 84 |
| DT- and ODT-Ag NPs | <50 | Toluene | 100 | 90 min; 900 and 7 | 0.13–0.16a | Gramicidin D, myoglobin, ubiquitin, and BSA | LLME-MALDI-MS | 85 |
| MUA- and ODT-Ag2Se NPs | 7–10 | Toluene | 100 | 60 min; 900 and 7 | 20–180c | Valinomycin, gramicidin D and hydrophobic proteins | LLME-MALDI MS | 86 |
| ODT-Pd NPs | 6.5 | Toluene | 100 | 60 min; 900 and 7 | 17–37c | Insulin, ubiquitin, lysozyme | LLME-MALDI MS | 87 |
| TiO2, NiO NPs and ODT – Pt NPs | 7.5–30 | Toluene | 100 | 5 min; 900 and 7 | — | Halobacterial caroteinods | LLME-TLC-MALDI MS | 88 |
| Ag NPs | — | CH2Cl2 and acetone | 100–200 | 5 min and 10 | 2–3c | Erythromycin, spiramycin, tilmicosin, and tylosin | DLLME-SALDI-MS | 89 |
| CTA-Co3O4 NPs | — | Toluene | 100 | 10 min; 900 and 7 | 5c | Insulin, chymotrypsinogen, lysozyme and milk proteins | LLME-MALDI MS | 90 |
| OA-Mg(OH)2 NPs | ∼30 | Toluene | 200 | 30 min; 900 and 7 | 2.0 and 24c | Valinomycin, gramicidin D, hydrophobic proteins in E. coli and B. subtilis | LLME-MALDI MS | 91 |
| HOA-BaTiO3 NPs | 30–40 | Toluene | 100–200 | 30 min; 900 and 7 | 0.20–0.40a | Phospholipids, hydrophobic proteins in E. coli | LLME-MALDI MS | 92 |
3.1 SDME Coupled with MALDI-MS for organic molecules assays
The screening of organic contaminants is of great importance in environmental and biological samples. The SDME directly coupled MALDI-MS is one of the most commonly used techniques for the identification of organic contaminants in environmental and biological samples. The performance of SDME coupled with MALDI-MS was illustrated by the identification and quantification of drugs in environmental and biological samples, Dasgupta and Liu in 1995.26 When SDME is coupled with MALDI-MS, the following characteristics should be possessed by the solvent which allows it to act as the best solvent: (i) it should have the ability to produce homogeneous crystals with the matrix that can provide efficient MALDI mass spectra; (ii) it should have a moderately volatile nature during droplet drying on the MALDI plates; (iii) it should be water-immiscible; and (iv) it should exhibit a moderate viscosity to clench the microdrop at the needle tip. In 2005, our group developed several SDME techniques coupled with atmospheric pressure (AP)-MALDI- and MALDI-MS for the quantification of organic compounds in environmental and biological samples.37–39 Briefly, SDME techniques were successfully used to extract and to preconcentrate monensin in soil, surface water and human urine prior to AP-MALDI-MS.37 In this method, three binary solvents (hexane–chloroform, 1-octanol–chloroform, and toluene–chloroform) have been used for the efficient extraction and preconcentration of monensin. Among these binary solvents, the chloroform–toluene system was effectively used to extract and preconcentrate monensin from the sample solution. To confirm the efficiency of the SDME-AP-MALDI-MS technique, collision-activated dissociation (CAD) experiments were performed for the sodiated monensin ion. It was found that the MS/MS of sodium adduct ion [M + Na]+ at m/z 693 yielded product ions at m/z 675, 657, 639, 599, 581, and 461, which confirmed the presence of monensin in the soil sample (Fig. 3). The developed system was successfully used to quantify monensin in soil and urine samples using a dibenzo-crown ether as an internal standard. The SDME-AP-MALDI-MS techniques proved to be a rapid and selective method for the determination of monensin in environmental and urine samples.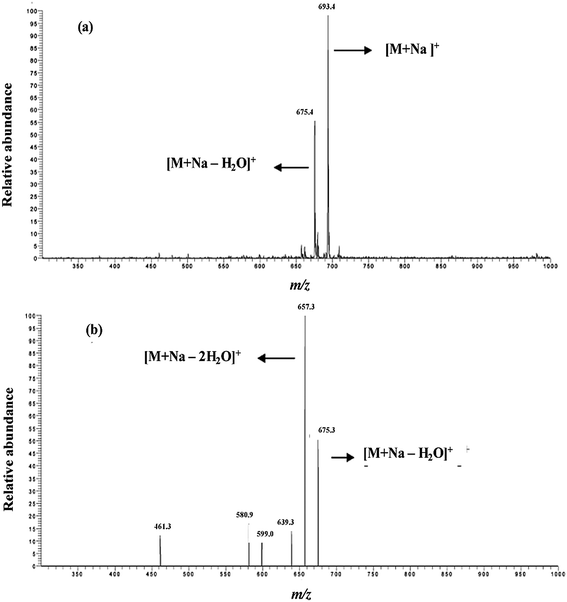 | ||
| Fig. 3 AP-MALDI mass spectra of monensin extracted using SDME: (a) monensin and (b) MS-MS product ions of m/z 693 obtained using CHCA as the matrix. Reprinted with permission from ref. 37. Copyright 2006 American Chemical Society. | ||
A dynamic drop-to-drop solvent microextraction was performed by using m-xylene (4.0 μL) as the extracting solvent for the extraction and preconcentration of quinine from a 30 μL sample.38 The extracted quinine was successfully identified by AP-MALDI-MS. Several influencing parameters such as the effect of solvent selection, sample volume, aqueous phase volume, organic phase volume, the addition of salt, and the pH were studied for the most efficient extraction of quinine from urine and plasma samples. Despite the power of the AP-MALDI-MS technique, it has disadvantages such as strong interference of matrix clusters in the low mass region <500 Da. To suppress the matrix cluster ions in the low mass region, our group developed a SDME technique for the extraction of drugs (amitriptyline – AM, nortriptyline – NTP, quinine – QN, imipramine – IP, trimeprazine – TMP) from sample solutions using 1.0 μL of xylene.39 The extracted drop was mixed with methyltrioctyl ammonium chloride as an additive along with α-cyano-4-hydroxycinnamic acid (CHCA) to reduce background noise in the low mass region. This method successfully generated analyte mass peaks at m/z 264.2, 278.2, 281.2, 298.1 and 325.3, which corresponded to protonated ions of NTP, AM, IP, TMP and QN, respectively. Additionally, these approaches provided a good linearity with the limit of detection (LOD), ranging from 0.18 μM to 18.3 μM for the above analytes. We calculated the enrichment factor (EF) to determine the extraction efficiency of the drugs under the optimized conditions. The EF values were found to be 13–52- and 5–13-fold for all drugs at a concentration of 1.6 mM, with and without MTOAC, respectively.
Cationic surfactants (CS) are surface-active compounds and contain at least a hydrophobic alkyl chain and a hydrophilic group carrying a positive charge. These are derived from natural oils and are extensively used as additives in hair rinses, textile softeners, preservatives, or antiseptic agents in industrial and commercial products. Due to their extensive use in various applications, CS substances are still present in many environmental samples. In order to estimate the concentration of these substances, we developed a SDME technique for the isolation and preconcentration of CS substances (cetylpyridinium chloride – CPC; methyltrioctyl ammonium chloride – MTOAC; cetyltrimethyl ammonium bromide – CTAB and tetraoctyl ammonium bromide – TOAB) in water samples (river and municipal wastewater).40 This method showed good recoveries ranging from 91.0 to 103.6%, with relative standard deviations (RSD) of 8.9–12.0% for river and municipal wastewater, respectively. We observed impressive enrichment factors in the range of 40–64-fold for all analytes in deionized water. To demonstrate the extraction efficiency of the SDME technique, we compared the LODs and enrichment factors of SDME with LODs and enrichment factors of hollow-fiber liquid-phase microextraction (HF-LPME) coupled to AP-MALDI-MS. The SDME coupled with AP-MALDI-MS approach provided good enrichment factors in the range of 40–64 for all analytes. Furthermore, our group also demonstrated the feasibility of direct coupling of immersed SDME technique directly combined with AP-MALDI-MS for rapid and sensitive analysis of hydrophilic drug (dopamine) in urine sample.41 In this method, several water-immiscible organic solvents such as toluene, octanol, ethyl acetate, isopropyl ether, and n-hexane were investigated as solvents for the isolation and preconcentration of dopamine in aqueous/urine samples. Among these, octanol acted as the best solvent because of its ability to form hydrogen bonds with the hydroxy groups of dopamine. This extraction mechanism was based on the hydrogen bonds between octanol and dopamine, which offers a detection limit for dopamine as low as 25 ppm in human urine. When compared to liquid–liquid extraction (LLE) and LPME using a dual gauge microsyringe with a hollow fiber (LPME/DGM-HF) coupled with AP-MALDI-MS, SDME-AP-MALDI-MS provided a better sensitivity for the high throughput analysis of dopamine. In order to explore the SDME for tandem mass studies, AP-MALDI-MS/MS was carried out on the protonated mass peak of dopamine at m/z 154 ([M + H]+). As a result, the protonated mass peak yielded two product ions at m/z 137, 119 and 91, corresponding to the loss of NH3 ([M + H–(NH3)]+), further loss of water and CO from the mass peak of 137. This approach provided strong evidence for the identification of dopamine in biological samples.
In recent years, there is growing interest in the development of new miniaturized analytical methodologies and SDME interfaces for the direct coupling with a mass spectrometer, leading to improvements in the efficiency/sensitivity of the technique. These developments have been aimed not only at completely new designs but also at the possibility of introducing extra phase means for SDME techniques with three-phase, which includes their miniaturization and automation as a result of the simplified SDME direct coupling with MS. Work to design new SDME techniques directly coupled with desorption/ionization mass spectrometers is underway. For example, Chen et al. developed a three-phase SDME technique directly coupled with a desorption electrospray ionization-mass spectrometric (DESI-MS) method for the trace detection of methamphetamine (MA) in aqueous solution.42 This three-phase SDME involves three steps, (1) hexamine was added to the sample tube with occasional shaking for 5 min and then stirred for 15 min at 1100 rpm to accelerate the hexane extraction of the analyte from the aqueous phase; (2) a 4 μL droplet of 5% acetic acid in water was suspended in the organic layer of hexane using a 50 μL flat-top HPLC syringe (Hamilton) inserted through an aluminum foil seal; (3) the solution was stirred at 1100 rpm for 40 min to extract MA into the droplet. The handing droplet was withdrawn back into the syringe and then directly coupled with DESI-MS for identification of MA (Fig. 4). The authors also performed two-phase liquid SDME to directly extract the product of an organic reaction performed in a room temperature ionic liquid (i.e., the nucleophilic addition of aniline to phenyl isothiocyanate to form N,N′-diphenylthiourea in 1-butyl-3-methyl-imidazolium tetrafluoroborate). The resulting reaction product droplet was directly injected to DESI-MS to examine the reaction products, which allows to develop a green analytical method. The DESI mass spectrum showed mass peaks at m/z 74 and 150, corresponding to the tetramethylammonium cation N(CH3)4+ from the internal standard and the protonated MA, respectively (Fig. 5). Recently, Bai's group described a rapid, simple, and efficient method for determination of six phytohormones (indole-3-acetic acid – IAA, indole-3-butyric acid – IBA, jasmonic acid – JA, salicylic acid – SA, abscisic acid – ABA, and gibberellin A3 – GA3) in fruit juices (pineapple, pear, and watermelon) by single-drop liquid–liquid–liquid microextraction (SD-LLLME) combined with direct analysis in real-time mass spectrometry (DART-MS).43 In both techniques,42,43 the parameters such as selection of organic phase, drop volume, pH, extraction time, pKa value, and solubility were optimized for the ideal SDME conditions. With these SDME developments, SDME coupled with DESI- and DART-MS approaches are mechanically and practically strong enough to extract and to preconcentrate trace target species from complex samples. Using these approaches, good extraction efficiencies and detection sensitivities were achieved and provided an effective platform for the rapid analysis of trace level target species in complex matrices.
 | ||
| Fig. 4 Schematics showing (a) the three-phase SDME set-up for MA analysis and (b) the two-phase SDME set-up for DPTU detection from an ionic liquid; (c) image showing the direct desorption and ionization of the obtained single droplet extract contained in the syringe with a needle of the deactivated fused silica capillary. Reproduced with permission from ref. 42. | ||
 | ||
| Fig. 5 DESI-MS spectra of three extraction replicates (a single scan is displayed for each spectrum of three replicates A–C). Inset shows the CID MS/MS spectrum of the protonated methamphetamine (MA) ion [MA + H] + (m/z 150). Reproduced with permission from ref. 42. | ||
In order to enhance the extraction efficiency and to minimize matrix clusters in the low mass region, Xie and co-workers developed a single drop extraction/derivatization method for the isolation and preconcentration of small molecular aldehydes (formaldehyde – FA, acetaldehyde – AA, acrolein – AL, crotonaldehyde – CA, 2-furaldehyde – 2-FAD and benzaldehyde – BA) in single puff smoke.44 In this method, a methanol solution of diphenylamine (DPA) and 2,5-dihydroxybenzoic acid (2,5-DHB) was used as the solvent for the extraction/derivatization of small molecular aldehydes (SMAs) and then this microdrop containing the ionic derivatization products of the SMAs could be directly deposited in the MALDI target to perform the measurement of SMAs in the cigarette smoke on the puff level by matrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance mass spectrometry (MALDI-FTICR-MS). This system successfully generated accurate derivatized small molecular aldehydes mass peaks at m/z 182, 196, 248, 206 and 258, corresponding to formaldehyde, acetaldehyde, 2-furaldehyde, acrolein and benzaldehyde, respectively. This method was linear in the range of 0.1–1.2 and 0.5–1.7 μg mL−1 for formaldehyde and acrolein, respectively. Authors reported impressive LODs at fg mL−1 for all analytes using a sample volume of 2.0 μL. With these new technologies, the ultra-trace levels of the target species were successfully extracted and preconcentrated and, consequently, enhanced the sensitivity of MALDI-MS. Importantly, these methods effectively reduced the volumes of solvents and samples required and allowed sample extraction and preconcentration in a single step.
3.2 SDME coupled with MALDI-MS for bioanalysis
Among the liquid-phase microextraction techniques, SDME remains one of the most powerful and versatile sample cleanup and preconcentration techniques, particularly for the analysis of biomolecules by MALDI-MS. This is due to the drop volume used in SDME being ideal and it can be directly combined with MALDI-MS for the identification of biomolecules in complex samples. In SDME, the solvent and sample volumes were greatly reduced from mL to μL (∼1.0 μL for drop and 100 μL for sample) and facilitated the automation of the process in direct conjunction with MALDI-MS analysis. So far, various modes of extraction have been introduced to isolate and to preconcentrate target analytes from samples (Fig. 2). Among these modes, only the direct immersion SDME was in practice directly conjugated with MALDI-MS. Our group described the utility of SDME directly combined with MALDI-MS for the direct analysis of hydrophobic peptides (valinomycin and gramicidin D) from biological samples (urine and plasma) in a high salt solution.45 In this method, we selected octanol as the best solvent for the extraction and preconcentration of hydrophobic peptides under high salt conditions, since it has a high immiscibility with aqueous phase (sample solution), moderate viscosity, and high extraction efficiency. This method showed a high tolerance capability for the efficient extraction and preconcentration of hydrophobic peptide (gramicidin D) at a salt addition of 1.7 M of NaCl and produced a good mass spectrum with enhanced signal intensities of the sodiated and potassiated ions of gramicidin D, since gramicidin D has showed an ubiquitous nature to interact with alkali ions with high affinity.46 Therefore, the sodium and potassium adduct ions of gramicidin D ([GrA + Na]+) and ([GrA + K]+) were generated with higher intensities than the protonated ion of gramicidin D ([GrA + H]+). Under optimal conditions, the signal intensities of the analytes were greatly increased up to 53-fold, which allowed the detection of the analyte at 130 nM in a high salt solution. Interestingly, the SDME-AP-MALDI-MS technique had a greatly improved sensitivity, 11–320 times that of the conventional AP-MALDI-MS. The method was applied to the analysis of β-carotine in carrots in a high salt solution.Even though it has the potential to isolate and preconcentrate biomolecules in high salt solutions, little work has been carried out on the direct use of an organic drop in SDME coupled with MALDI-MS for bioassays. Most of the SDME-MADLI-MS studies to date have described the integration of nanomaterials in SDME to capture analytes and to act as energy mediators (absorb laser light to transferring its energy to the analytes), which allows their effective desorption/ionization during the MALDI-MS for a wide variety of molecules analysis. Therefore, in the next section we would like to explore the beauty of nanomaterial-integrated SME (SDME and LLME) techniques coupled with MALDI-MS for organic and biomolecule assays in various sample matrices.
3.3 LLME coupled with MALDI-MS for organic and biomolecule assays
Current trends in green analytical chemistry are highly focused on miniaturized solvent extraction techniques for the simplification and automation of the entire sample pretreatment procedure prior to use in the analytical instrumentation. The development of simplified and miniaturized liquid–liquid extraction methods are based on the use of minimum volumes of solvents and samples that can be easily combined with MALDI-MS. On the way to achieve the efficient extraction of target analytes at ultra-trace levels, several efforts have been made towards the miniaturization and simplification of sample-preparation procedures. These advances have focused on minimizing the volumes of both samples and solvent, reagent consumption, while maintaining high selectivities and recoveries and using environmentally friendly methods, which is currently considered as the rate -determining step for green analytical processes.To date, only the LLME mode is widely used for direct coupling with MALDI-MS for the analysis of organic molecules in various samples. For example, Guo et al., described the utility of headspace liquid-phase microextraction (HS-LPME) coupled with matrix-assisted laser desorption/ionization-Fourier transform mass spectrometric (MALDI-FTMS) method for the analysis of volatile basic components in tobacco.47 In this method, 25 volatile basic compounds were successfully isolated by using the solvent composition TFA + H2O + acetone + 2,5-DHB in basic medium, and were accurately identified by FTMS. It was confirmed that HS-LPME-MALDI-FT-MS is clearly far superior to LPME-GC-MS. The same group reported another use of LPME coupled with MALDI-FTMS for the measurement of nicotine content in mainstream smoke at the single puff level.48 They used 2,5-DHB (dissolved in acetone with 1% TFA) and glycerol as the multifunctional solvent (as solvent, as additive and as matrix) for the extraction and preconcentration of nicotine in mainstream smoke. As a result, this method showed good reproducibility, and a good quality of MALDI mass spectrum was produced by using glycerol as an additive, which allows the homogeneous distribution of the analyte. This method showed good linearity, offering a LOD of 0.12 nM.
To overcome the problems usually associated with the identification of lipopolysaccharides in bacteria, new procedures are being published that use microscale extraction coupled with mass spectrometry to increase the precision and accuracy of the bioanalytical methods. Caroff's group develop a novel and rapid strategy for the microscale extraction of lipopolysaccharides (endotoxins, LPSs) from rough-type Gram-negative bacteria (Escherichia coli O119 strain 19392, Bordetella bronchiseptica 4098 and Bordetella parapertussis strain 15989) by using a modified enzyme–phenol–water system as solvent.49 The extracted LPs were successfully separated and identified using thin-layer chromatography (TLC) combined with MALDI-MS. To improve the quality of the MALDI mass spectra, the authors examined several acids (citric, malonic, oxalic, succinic, and tartaric) and their salts along with 2,5-DHB as the matrix. Among these, citric acid (0.1 M) with the 2,5-DHB system greatly improved the LPs mass spectra. As a result, several mass peaks at m/z 1163, 1333, and 1572, corresponding to the tri-, tetra-, and penta-acylated fragmented species of the lipids, and at m/z 1442, 1504, 1664, and 1706, corresponding to the oligosaccharides in B. parapertussis. Similarly, few mass peaks are observed at m/z 1797 and 1571, which related to hexaacyl lipid A and pentaacyl lipid A, respectively. Janfelt and co-workers developed a hollow fibre liquid-phase microextraction coupled with desorption electrospray ionization mass spectrometric (DESI-MS) method for the identification and quantification of basic drugs (diphenhydramine – DPA, loperamide – LA, methadone – MA, NTP, AM, and pethidine – PI) in human urine samples.50 They performed three-phase (hollow fibre was dipped for 2 s into 1-octanol + 10 mM HCl as acceptor) and two-phase (hollow fibre was dipped for 2 s into toluene and then filled with 10 μL of toluene) microextractions for the extraction of basic drugs prior to their identification by DESI-MS. Even though both extraction systems could improve the signal intensities of basic drugs in DESI mass spectra, the three-phase LPME provided a superior sample cleanup to the two-phase. As a result, good rectilinear relationship was obtained between the amount of basic drugs and their signal intensities in the range 50–2000 ng mL−1 (R2 = 0.992), with LODs in the range 25–100 ng mL−1, respectively. This innovation appears to be a promising analytical tool for the monitoring of basic drugs in biological fluids with the excellent selectivity and the high speed of DESI. However, the limitation of this method is the hollow fibers can not be reused.
During the past 50 years, pesticides (organophosphorus, carbamates, organochlorines and neonicotinoids) have been used in increasing amounts in various agriculture sectors throughout the world. These are a wide group of polluting compounds with anthropogenic origin that are used as insecticides, fungicides, neonicotinoids and herbicides. Among these, neonicotinoids, also known as chloronicotinyls, are a group of insecticides with a wide range of chemical and biological properties, which can be used for crop protection and in veterinary medicine.51 Due to their ultra-trace contaminations in various sample matrices, the development of precise, accurate and ultra-sensitive analytical methods, associated with simplicity and celerity, is still a hard task to undertake. To overcome this problem, the ultra-trace target species must be extracted and preconcentrated prior to their identification by analytical instruments. To achieve high recoveries and efficient sample enrichments, Hernández-Córdoba's group developed a new solvent microextraction technique through the combination of solid-phase extraction with dispersive liquid–liquid microextraction (SPE-DLLME) for the determination of five neonicotinoid insecticides (acetamiprid – ACE, thiamethoxan – TMX, thiacloprid – THIA, clothianidin – CLO and imidacloprid – IMI) in honey.52 In this approach, CHCl3 was used as the extracting solvent and the extraction was carried out at 10% (w/v) NaCl. The five neonicotinoid insecticides were successfully extracted and preconcentrated within minutes and then identified by atmospheric pressure chemical ionization-ion trap-tandem mass spectrometry (APCI-IT-MS/MS). The calibration graphs were linear in the range of 0.1–7500 ng g−1 with the detection limits 0.02–1.0 ng g−1 for five analytes.
Our group also developed a new LPME method using a micropipette with disposable tips that was directly coupled with atmospheric pressure MALDI-MS (AP-MALDI-MS) for the extraction and preconcentration of nortriptyline in human urine and plasma samples.53 The extraction was performed using a micropipette (0.5–10.0 μL) with a hole size of 0.61 mm (i.d.). The analysis of nortryptyline was performed in full scan, selective ion monitor (SIM), and selective reaction monitor (SRM) of MS/MS. The best mass spectra were obtained using 1000 ppm of CHCA as the matrix. It was found that the MS/MS method provided a wider linear range and lower LODs, however, poorer relative standard deviation (RSD) values were observed than for the full scan and SIM methods. The quantitative analysis was carried out by monitoring ions at m/z 264 for full scan and SIM, and ion at m/z 233 MS/MS, respectively. The base peak at m/z 233 ([M + H–CH3NH2]+) was generated by the loss of CH3NH2 from the protonated nortryptyline. The enrichment factor (36.5–43.0 fold) was obtained from a 20 mL of sample solution, while, the LODs were found to be 6.26, 47.5, and 94.9 nM for water, urine and plasma, respectively. This innovation appears to be a promising analytical tool for monitoring of nortryptyline in biological and clinical samples.
Liquid-phase microextraction is fast, selective and a relatively inexpensive solvent free sample-preparation technique. Despite many benefits of LPME for bioanalysis, limited papers have described the application of LPME directly coupled with MALDI-MS in the biological field. As a result, the majority of liquid-phase microextraction research works coupled with various analytical instruments (UV-visible, AAS, CE, ICP-MS) have already been performed for inorganic and organic molecule assays,7–21 however, in the last decade their potential for the extraction and preconcentration of trace biomolecules has been recognized. To date, only a few papers are devoted to the analysis of biomolecules using LPME directly coupled with MALDI-MS from biological fluids. For example, Sweedler's group described the potential of liquid microextraction coupled with MALDI-MS for the extraction, concentration and detection of a complete set of peptide transmitters in a single cell.54 They used 2,5-dihydroxybenzoic acid with a mixture of acetone and water as the solvent system for the extraction of trace-level gene expression products in single cells. Using this approach, Aplysia californica B1 and B2 motor neurons were effectively identified in single cells with minimal sample volume and sample preparation procedures. This method was proved to be a multidisciplinary approach for single-cell MALDI-MS peptide profiling, northern analysis, in situ hybridization, and immunocytochemistry, which allows the characterization of a more complete set of neurotransmitters in neurons.
Furthermore, Ho et al., described a method for the extraction and preconcentration of peptides and digested proteins (bovine serum albumin – BSA, lysozyme, myoglobin and ovalbumin) using a ternary component system (CHCA/3-aminoquinoline (3-AQ)/quinoline (Q); at a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as the extracting solvent as well as a liquid matrix for MALDI-MS analysis.55 The extraction mechanism is based on the hydrophobic interactions between the peptides and liquid matrix. Since the multicomponent system has a hydrophobic as well as ionic nature due to the reaction between the acidic CHCA and basic 3-AQ, it allows it to act as an ionic solvent to extract peptides according to the “like dissolves like” rule. This method was successfully applied to extract peptides (angiotensin I) and trypsin digests (BSA, lysozyme, myoglobin and ovalbumin) in the presence of signal suppressing buffers and denaturants. Fig. 6 shows the MALDI mass spectra of the tryptic digest of BSA (0.36 μM in 25 mM Tris and 2 M urea) before and after LLME. It was noticed that a higher number of digested protein peaks were successfully generated with high signal intensities, which corresponds to the peptides of BSA. The ensuing LOD for angiotensin I was 1.25 nM, and the digested protein sequences were effectively observed in the one-step procedure (sample concentration and clean up). Based on the obtained results, the authors found that the performance of the LLME technique is somewhat less than that obtained from direct MALDI-MS. Table 2 summarizes the LLME techniques directly coupled with mass spectrometric techniques for the analysis of organic and biomolecules.
4) as the extracting solvent as well as a liquid matrix for MALDI-MS analysis.55 The extraction mechanism is based on the hydrophobic interactions between the peptides and liquid matrix. Since the multicomponent system has a hydrophobic as well as ionic nature due to the reaction between the acidic CHCA and basic 3-AQ, it allows it to act as an ionic solvent to extract peptides according to the “like dissolves like” rule. This method was successfully applied to extract peptides (angiotensin I) and trypsin digests (BSA, lysozyme, myoglobin and ovalbumin) in the presence of signal suppressing buffers and denaturants. Fig. 6 shows the MALDI mass spectra of the tryptic digest of BSA (0.36 μM in 25 mM Tris and 2 M urea) before and after LLME. It was noticed that a higher number of digested protein peaks were successfully generated with high signal intensities, which corresponds to the peptides of BSA. The ensuing LOD for angiotensin I was 1.25 nM, and the digested protein sequences were effectively observed in the one-step procedure (sample concentration and clean up). Based on the obtained results, the authors found that the performance of the LLME technique is somewhat less than that obtained from direct MALDI-MS. Table 2 summarizes the LLME techniques directly coupled with mass spectrometric techniques for the analysis of organic and biomolecules.
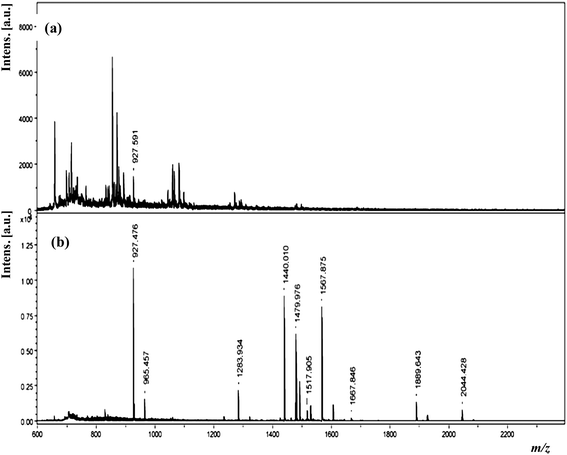 | ||
| Fig. 6 MALDI mass spectra of tryptic digest of BSA (0.36 μM in 25 mM Tris and 2 M urea) (a) before and (b) after LLME. Labeled peaks correspond to the matched peptides of BSA. Reprinted with permission from ref. 55. Copyright 2010 American Chemical Society. | ||
4 Liquid-microjunction extraction coupled with MS techniques
It is well known that MS-based technologies are widely used in modern biology to meet the challenges of proteomics after much adaptation.56 This is due in part to the forced blending of the aforementioned miniaturized-based analytical methodologies with MS tools adapted from the ‘proteomics toolbox’, which allows to elucidate peptide/protein sequences and/or structure with minimal volume samples.57 However, protein precipitation falls short in many circumstances, depending upon the molecular weight of the peptide/protein and the nature of the biological matrix. To address these challenges, in 2002 Van Berkel's team introduced a liquid microjunction surface sampling probe (LMJSSP) that can be easily coupled with MS tools for the monitoring of metabolites and proteins in tissue samples.58 Basically it is a liquid extraction probe that allows the extraction of analytes from a surface followed by MS analysis. In this approach, the analyte is reconstituted from the surface by connecting the probe, which is composed of two channels with a wall-less liquid microjunction. The liquid flow rate is controlled by the aspirating rate from the nebulizing gas of the pneumatically assisted ionization probes (ESI or APCI). Therefore, it is included in the genre of liquid extraction based surface sampling techniques for MS.For the direct analysis of spotted and dosed drugs and their metabolites in tissues, Van Berkel et al. developed a series of liquid microjunction surface sampling probes coupled with MS tools.58–64 It proved as a proof-of-principle sampling technique in the direct analysis of sulforaphane and its glutathione and N-acetyl cysteine conjugates in the stomach and various other tissues from the dosed mice without the need for sample preparation.59 They also described the use of a liquid extraction based sealing surface sampling probe for the direct mass spectrometric analysis of drugs (sitamaquine or acetaminophen) and their metabolites in dried blood spots and whole mouse thin tissue sections.60 Using this approach, propranolol and its hydroxyproranolol glucuronide metabolite were effectively quantified in specific organ tissues. The results agreed well with whole-body autoradiography, and high-pressure liquid chromatography (HPLC)-mass spectrometry. To enhance the rapidity of the LMJ-SSP method, they designed a LMJ-SSP probe by alignment of the probe and surface distance at <20 μm for the successful surface sampling of tissues in the high-throughput quantitative analysis of drugs by ESI-MS.61 This method was successfully applied to extract and to detect two isomeric phase II metabolites of propranolol (an aliphatic and an aromatic hydroxypropranolol glucuronide) in four different mice organs (brain, lung, kidney, and liver) with good selectivity and sensitivity.62 To extend the application of LMJ-SSP, the authors developed a fully automated liquid extraction-based surface sampling device combined with an Advion NanoMate chip-based infusion nanoelectrospray ionization MS method for the monitoring of drugs and metabolites in the tissues of dosed mice.63 They studied the performance of a sampling probe by combing with three types of analytically important sample surface types such as spotted sample arrays on a MALDI plate, dried blood spots on paper, and whole-body thin tissue sections from drug dosed mice. Recently, the same group developed a continuous-flow liquid microjunction surface sampling probe coupled with HPLC-ESI-MS for the extraction, separation and detection of small molecules and proteins from surfaces in spatially resolved (∼0.5 mm) tissues.64 They achieved impressive extraction recoveries within 40 s and confirmed the presence of the parent drug and two different hydroxypropranolol glucuronides in the tissues. Based on these results, these approaches were proved to be one-step sampling probes for the assignment and quantification of different molecules by MS tools in various tissue sections.
The analysis of molecular components in tissues plays a key role in the understanding of cellular mechanisms, their regulation and the physiological processes of cells in living organisms. The cellular changes have a great influence on the regular functions of proteins and these changes have a significant impact on their regulation (activation/repression of protein synthesis), primary structure, spatial arrangement and ability to complex with other partners (protein concentrations and primary structure or due to changes in the environment such as ionic strength or pH).65 To resolve these drawbacks, the Salzet's group developed a new liquid microjunction coupled with a MALDI mass spectrometric imaging (MSI) strategy for the protein identification of discrete regions of tissue sections that avoids the dilution of low abundant proteins.66 Using this approach, almost 1500 proteins were successfully identified with high confidence and good reproducibility for a given position spot at 670 μm in diameter, which consists of about 1900 cells in the tissue section (Fig. 7). The authors applied this probe for on-tissue enzymatic digestion followed by micro-extraction and subsequent analysis by MALDI-MSI and these results have allowed the indirect back identification and subsequent confirmation of the protein distribution in the tissues. They unambiguously identified proteins in tissues with high confidence and spectral resolution that should provide interesting insights for clinical applications.
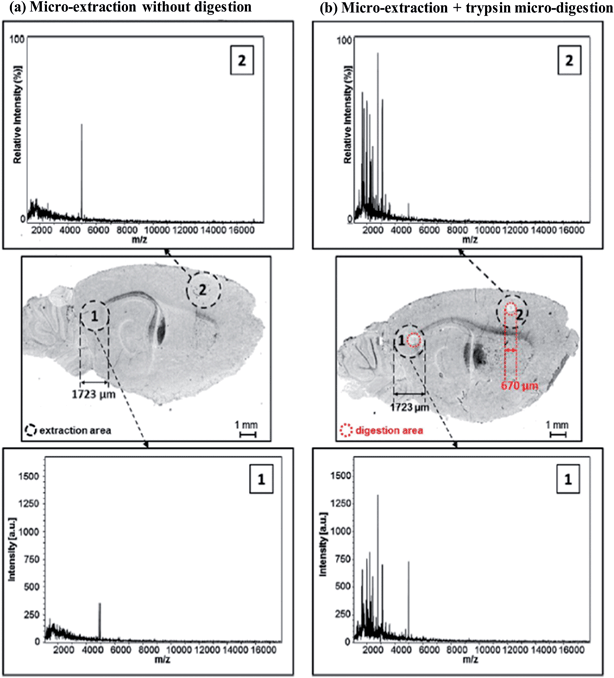 | ||
| Fig. 7 On-tissue liquid micro junction extraction of trypsin digested or undigested regions. Comparison of MALDI-TOF MS spectra recorded in the linear positive mode from 2 consecutive rat brain sagittal tissue sections at two distinct locations in two regions (1,2) of the tissue after micro-extraction in the optimized conditions for tryptic digestion peptides (a) micro-extraction of non-digested areas and (b) micro-extraction after on tissue digestion on 300 μm spots. Black circles delimit the micro-extraction areas which are about 771 μm diameter and red circles define the position of the 300 μm trypsin digestion spots. Reproduced with permission from ref. 66. | ||
5 Nanomaterial-assisted miniaturized solvent microextraction techniques coupled with MALDI-MS
In recent years, nanomaterials have been exploited in wide range of analytical applications including extraction and preconcentration of ultra-trace level target species prior to MALDI-MS. Nanomaterials have been proved to be potential candidates in MALDI-MS for addressing several problems such as the suppression of background noise, low mass analytes, and the enrichment of low abundant species signal intensities from various matrices.67,68 Nanomaterials are attractive because of their unique physico-chemical properties (size, composition, conductivity, magnetism, mechanical strength, light absorbing, and emitting properties). As a result, various nanomaterials have been used as matrices,69–72 as affinity probes,73–75, as extracting probes76–80 for the analysis of biomolecules by MALDI-MS. Furthermore, great efforts have been devoted on the integration of nanomaterials in MALDI-MS for bioanalysis since its invention by the Tanaka group,4 who described the use of cobalt particles (∼30 nm) suspended in glycerol for the ionization of large biomacromolecules. Apart from these applications, nanomaterials have also been used in miniaturized solvent extractions for sample clean-up and the preconcentration of analytes prior to MALDI-MS. By utilizing nanomaterials, the signal intensities of ultra-trace target species have been greatly enhanced in the mass spectra because the materials are used to either capture/concentrate the target species or amplify the signal associated with detection. Therefore, there has been a significant increase in the number of publications over the last decade in which nanomaterials have been employed as extracting and preconcentrating probes in SDME and LPME techniques coupled with MALDI-MS. In this part, we will highlight the recent advancements in the use of nanomaterials in miniaturized solvent extraction (SDME and LMPE) techniques coupled with MALDI-MS for target analyte assays.5.1 Nanoparticle-assisted SDME coupled with MALDI-MS
The unique characteristic structures and physico-chemical properties of metal nanoparticles (NPs) allow them to interact strongly with ultra-trace target species, via electrostatic and hydrophobic interactions. These interactions and high surface nano-sized structures make them good candidates for extracting and concentrating probes in miniaturized solvent extraction techniques. This will result in the effective and selective capture of analytes from biological complexes with minimal sample volumes. NPs not only assist in sample preparation, they also play important roles in determining the sensitivity of the MALDI-MS for molecular assays. They have the ability to capture the target species and potentially absorb the laser energy and then transfer it to the analytes for their desorption and ionization. NPs exhibit high absorptivity at the emission wavelength of laser irradiation, which allows the absorption of energy and the efficient transfer of it to the analytes. As a result, the mass peaks of target analytes were greatly enhanced with reduced background noise in the low mass region.Although most of the miniaturized solvent extraction techniques (SDME and LPME) have been developed over the last two decades, the first metal NPs integrated SDME technique coupled with MALDI-MS was reported by our group in 2005.81 In this method, tetraalkylammonium bromide (TAAB) functionalized Au NPs were dispersed in toluene and then used as solvent in SDME coupled with atmospheric pressure matrix-assisted laser desorption/ionization mass spectrometry (AP-MALDI-MS) for the extraction, preconcentration and analysis of peptides such as methioone-enkephalin (Met-enk) and leucine-enkephalin (Leu-enk) from urine samples. The extraction and preconcentration mechanisms were based on the electrostatic interactions between the Au NPs and peptides. Biomolecules (peptides and proteins) exhibit specific isoelectric points (pI); below the isoelectric point they exhibit a positive charge and vice versa. Using this approach, the mass peaks at m/z 556.17 and 574.2 correspond to protonated ions of Leu- and Met-enk, respectively. This method was successfully used to extract and preconcentrate target species in the presence of Triton X-100 (nonionic surfactant) and urea (major matrix interferences) as matrix interferences at a sample volume <25 μL. We also carried out MS/MS on protonated Met-enk (m/z 574.1) and yielded product ions such as [Met-enk-H2O + H]+ (m/z 556), b4 (m/z 424.93), a4 (m/z 397.01), y3 (m/z 353.91), and b3 (m/z 424.93). Furthermore, the same MS/MS was also performed on the protonated Leu-enk (m/z 556.0) and yielded fragment ions such as [Leu-enk-H2O + H]+ (m/z 538.05), b4 (m/z 424.93), a4 (m/z 397.01), y3 (m/z 335.87), and b3 (m/z 278.88), respectively. To enhance the applicability of the method, we formed organic frameworks on the Au NPs to use them as multifunctional probes (as a binary matrix, as extracting and preconcentrating) in SDME combined with MALDI-MS for the nalysis of peptides and trypsin digest proteins.82 The organic framework was formed by synthesis of (4-mercaptophenyliminomethyl)-2-methoxyphenol (Schiff base 3; MPIMP) using vanillin, ethanol, acetic acid and 4-aminothiophenol as precursors followed by capping it on the Au NPs surfaces. The engineered Au NPs acted as both signal enhancers and target concentrators, enabling the very rapid and direct detection of various biomolecules including gramicidin D (53.1 fM), HW6 (450 fM), ubiquitin (23.3 fM), cytochrome c (40 fM) and myoglobin (35.3 fM) by MALDI-MS.
Another similar approach was described for the extraction and preconcentration of peptide mixtures from biological samples (urine and plasma) using functionalized Ag NPs-integrated SDME.83 In this approach, Ag NPs were functionalized with tetraoctylammonium bromide (TOAB) and then used as extracting probes for the extraction and preconcentration of peptides through electrostatic interactions between the positive charges of TOAB on the surfaces of the Ag NPs and the negative charges of the peptides. As a result, this system successfully minimized unwanted effects (background noise), the dilution of the sample or washing of the deposited sample, which permits the extraction of analytes even with high matrix interferences such as from 1% Triton X-100 and 6 M urea. To extend the application of SDME for bioanalysis, nanomaterials–ionic liquid hybrids or dispersions are used as solvent for SDME coupled with MALDI-MS for trace analysis of biomolecules. In our work, Pt NPs were dispersed in ionic liquid (1-butyl-3-methylimidazolium hexafluorophosphates, BMIHFP) and then used as a probe for the extraction and preconcentration of biomolecules in bacteria prior to their identification by MALDI-MS.84 The Pt NPs dispersed ionic solvent provides a flexible environment to facilitate specific targeting and efficient bacteria protein uptake from bacteria (Escherichia coli, and Serratia marcescens). The electrostatic and hydrophobic interactions play a key role in bacterial proteins extraction/preconcentration from bacteria, and offers bacteria protein detection at 106 cfu mL−1. These NPs-based SDME coupled with MALDI-MS approaches provide simple platforms for bioanalysis, offering detection limits as low as fM from sample volumes of 100–500 μL.
5.2 Nanomaterial-based LPME coupled with MALDI-MS
The unique properties (dispersion ability and high surface area to volume ratio) of nanomaterials play key roles in LPME techniques for the extraction and preconcentration of trace target species from sample matrices with minimal volumes of samples (μL). This technique is easily integrated with MALDI-MS for the analysis of a wide variety of chemical and biological systems with improved selectivity and sensitivity.85–92 By using these approaches some of the shortcomings of SDME can be overcome, such as drop instability, a limited ability to extract a higher number of analytes, and the avoided loss of target species during sample clean-up steps.To date, many papers have described the applications of engineered metal and metal oxide nanomaterials in LPME coupled with MALDI-MS for the extraction, preconcentration and analysis of various molecules from various sample matrices.85–92 We have applied functionalized metal NP-assisted LPME to the extraction and preconcentration of peptides and proteins for MALDI-MS analysis.85–87 Briefly, the dodecanethiol (DT)- and octadecanethiol (ODT)-capped Ag NPs were used as hydrophobic/affinity probes for the LLME of peptides (gramicidin D) and proteins (myoglobin, ubiquitin, and BSA) prior to their identification by MALDI-MS.85 It was observed that the ODT-Ag NPs exhibited a better extraction efficiency than that of the DT-Ag and bare Ag NPs. This is due to the large number of alkyl chains on the Ag NPs, which facilitate a higher hydrophobicity, and provide greater extraction efficiencies through hydrophobic interactions. Similarly, 11-mercaptoundecanoic acid (MUA) and ODT were functionalized with Ag2Se NPs and then integrated with LLME for the extraction of hydrophobic peptides (valinomycin and gramicidin D) and hydrophobic proteins from soybean, which were then identified by MALDI-MS.86 A key advantage of this approach was the extraction/preconcentration of hydrophobic proteins from soybean via hydrophobic interactions, and the observed mass peaks at m/z 3879, 7855 and 8570 correspond to soybean hydrophobic protein, soybean Bowman–Birk proteinase inhibitor and soybean inhibitor D-II, respectively. A similar approach has also been applied to the extraction and preconcentration of proteins (insulin, ubiquitin, lysozyme) using ODT-capped Pd NPs as the extracting probes.87 The proteins (insulin, ubiquitin and lysozyme) were effectively extracted at a sample pH of ∼ 6.5, because of the enhancement of hydrophobic interactions between the proteins and the hydrophobic ligands of the ODT-Pd NPs. As a result, detection limits of 17–37 nM were achieved.
Recently, our group described the use of ODT-capped Pt NPs-assisted LPME for the extraction and preconcentration of pigment molecules from halobacteria (H. mediterannei), which were then identified by MALDI-MS.88 Using this approach, several mass peaks were observed at m/z 620, 690, 723, 740, 885 and 932, corresponding to 2-isopentenyl-3,4-dehydrorhodopin, trianhydrobacterioruberin, monoanhydro-bacterioruberin, bacterioruberin, phosphatidyl glycerol phosphate, and phosphatidyl glycerol phosphate (containing the molecule pyranose sugar), respectively. Apart from these peaks, another peak at m/z 900 was assigned as the methyl phosphatidyl glycerol phosphate in H. mediterannei. The extraction and preconcentration were based on the interaction between ODT and acyclic structure of the isoprenoid chain in the target species, and thereby concentrates them on the nanoparticle surfaces. Chen et al. have integrated dispersive LMPE with Ag NPs-assisted laser desorption/ionization MS for the extraction and detection of macrolide antibiotics (erythromycin, spiramycin, tilmicosin, and tylosin).89 The authors observed that the composition of the extracting solvent, solvent volume and pH played key roles in the efficient extraction of macrolide antibiotics, and the resulting impressive detection limits were 2, 3, 3, and 2 nM for erythromycin, spiramycin, tilmicosin, and tylosin, respectively.
Furthermore, our group also described the utility of metal oxide nanomaterials such as Co3O4 NPs, Mg(OH)2 NPs and BaTiO3 NPs for the LPME coupled with MALDI-MS for bioanalysis.90–92 Briefly, Co3O4 NPs were functionalized with cetyltrimethylammonium (CTA) and used as probes for the efficient capture of proteins (insulin, ubiquitin, chymotrypsinogen and lysozyme) via electrostatic and hydrophobic interactions.90 This system was successfully used to extract proteins at 5.0 nM under high salt conditions (3% NaCl).
In our recent work, oleic acid (OA) capped Mg(OH)2 NPs91 and 12-hydroxy octadecanoic acid (HOA) capped BaTiO3 NPs92 were used as matrices, extracting and hydrophobic affinity probes in LLME for the identification of hydrophobic peptides (valinomycin and gramicidin D), phospholipids (PLs) and hydrophobic proteins from Escherichia coli and Bacillus subtilis in MALDI-MS. It was observed that the surface supramolecular chemistry on the Mg(OH)2 and BaTiO3 NPs played key roles in the enhancement of the scope of the miniaturized sample preparation for bioassays that can be targeted for the effective extraction and preconcentration of hydrophobic targets from the biocomplex samples prior to their identification by MALDI-MS. A key advantage of NPs surface chemistry is to extract and to preconcentrate target analytes at high salt conditions (NaCl) and surfactant media (Triton X-100), allowing the target analytes to be easily ‘read out’ by MALDI-MS with lower LODs values (2.0–4.0 nM – peptides and 2 × 108 cfu mL−1 – bacterial proteins). As a result, both the methods successfully extracted and preconcentrated the hydrophobic and membrane proteins from bacteria (Escherichia coli and Bacillus subtilis, 2 × 108 cfu mL−1) prior to their identification by MALDI-MS. The mass peaks at m/z 3.4, 7.2, and 10.8 kDa correspond to membrane proteins ecnB (P56549), lpp (P69776), and osmE (P23933), respectively. The other membrane proteins such as Braun's lipoprotein (lpp), major outer membrane protein, cell envelope lipoprotein entericidin B (ecnB) and water-insoluble ATPase proteolipid (atpL) were also identified in E. coli. Importantly, the hypothetical membrane proteins yifL (P39166), ygdI (P65292) and acetyl-acyl carrier protein (acetyl-ACP, P0A6A8) mass peaks were observed at m/z 5.8, 7.0 and 8.892 kDa (Fig. 8). Presumably, surface modified NPs-assisted microextraction approaches allow either the selective extraction of the target analyte(s) or selective rejection of interfering species, providing even more amplified signals in the mass spectra. The applications of NPs integrated solvent microextraction techniques coupled with MALDI-MS for analytes assays are summarized in Table 3.
 | ||
| Fig. 8 MALDI mass spectra of identified hydrophobic proteins in E. coli using (a) HOA-modified BaTiO3 NPs-assisted LLME along with SA (0.5 M) as the matrix and (b) SA (0.5 M) as the matrix without HOA-modified BaTiO3 NPs. Reproduced with permission from ref. 92. | ||
6 Conclusions and perspectives
In this review, we describe the recent developments of miniaturized solvent extraction tools directly coupled with ESI- and MALDI-mass spectrometric techniques, which have the potential to provide efficient extractions, preconcentrations and analyses of organic and biomolecules from various samples matrices. An overview of the current literature reveals that exhaustive efforts have been devoted towards solvent microextraction techniques (LPME and SDME) directly coupled with ESI- and MALDI-MS to extend their applications to organic and biomolecule assays from more complex matrices. These approaches have provided many advantages such as rapidity, minimal volumes of sample and solvents, and the enrichment of low abundant species in a single step. Furthermore, these studies have exhibited good recoveries and high enrichment factors with good precision. It was noted that several parameters such as the selection of solvent, extraction time, pH, drop volume (for SDME) and sample agitation time influence extraction and preconcentration efficiency of target species from sample matrices.The continual development and introduction of novel material-based SME demonstrates the advantages of their unique separation selectivity and efficiency in preconcentrating ultra-trace level target species. The application of nanomaterials in LPME and SDME has opened up a new avenue for achieving highly selective and efficient extractions due to the unique physico-chemical properties of nanomaterials. The integration of nanomaterials in SME techniques has tremendous promise in achieving high extractions, preconcentrations, and offering lowering detection limits. Not only as extracting or preconcentrating probes, nanomaterials also act as supporting matrices to enhance signal intensities with reduced background noise and also provide high shot-to-shot reproducibilities. However, new solvent- or nanomaterials-based SME techniques are needed to develop complete solutions for many separation problems prior to their direct analysis by ESI- and MALDI-MS. Therefore, the surface engineering of nanomaterials would enable the better tunability of interactions between NPs and biomolecules, leading to new applications in miniaturized extraction methods, opening up tremendously new avenues in separation and mass spectrometry communities.
References
- B. T. Chait, Annu. Rev. Biochem., 2011, 80, 239–246 CrossRef CAS PubMed.
- J. B. Fenn, M. Mann, C. K. Meng, S. F. Wong and C. M. Whitehouse, Science, 1989, 246, 64–71 CAS.
- M. Karas and F. Hillenkamp, Anal. Chem., 1988, 60, 2299–2301 CrossRef CAS.
- K. Tanaka, H. Waki, Y. Ido, S. Akita, Y. Yoshida and T. Yoshida, Rapid Commun. Mass Spectrom., 1988, 2, 151–153 CrossRef CAS PubMed.
- M. Kuster, M. L. Alda and D. Barcelo, Mass Spectrom. Rev., 2006, 25, 900–916 CrossRef CAS PubMed.
- Y. P. Ho and P. M. Reddy, Clin. Chem., 2010, 56, 525–536 CAS.
- S. K. Kailasa and H. F. Wu, in Inorganic contaminants: Sample Preparation Approaches in Comprehensive Sampling and Sample Preparation, ed. J. Pawliszyn, X. C. Le, X. F. Li and H. K. Lee, Elsevier, UK, 2012, vol. 3 Search PubMed.
- H. Kataoka, Anal. Bioanal. Chem., 2010, 396, 339–364 CrossRef CAS PubMed.
- H. Kataoka, Trends Anal. Chem., 2003, 22, 232–244 CrossRef CAS.
- F. M. Musteata and J. Pawliszyn, Trends Anal. Chem., 2007, 26, 36–45 CrossRef CAS PubMed.
- D. Han and K. H. Row, Microchim. Acta, 2012, 176, 1–22 CrossRef CAS.
- M. S. Chang, Q. Ji, J. Zhang and T. A. Shourbagy, Drug Dev. Res., 2007, 68, 107–133 CrossRef CAS PubMed.
- F. P. Pereira, I. Lavilla and C. Bendicho, Spectrochim. Acta, Part B, 2009, 64, 1–15 CrossRef PubMed.
- A. N. Anthemidis and K. I. Ioannou, Talanta, 2009, 80, 413–421 CrossRef CAS PubMed.
- M. A. Jeannot, A. Przyjazny and J. M. Kokosa, J. Chromatogr. A, 2010, 1217, 2326–2336 CrossRef CAS PubMed.
- J. M. Kokosa, Trends Anal. Chem., 2013, 43, 1–13 CrossRef PubMed.
- H. Yan and H. Wang, J. Chromatogr. A, 2013, 1295, 1–15 CrossRef CAS PubMed.
- L. Kocúrová, I. S. Balogh, J. Šandrejová and V. Andruch, Microchem. J., 2012, 102, 11–17 CrossRef PubMed.
- C. Nerín, J. Salafranca, M. Aznar and R. Batlle, Anal. Bioanal. Chem., 2009, 393, 809–833 CrossRef PubMed.
- Y. Chen, Z. Guo, X. Wang and C. Qiu, J. Chromatogr. A, 2008, 1184, 191–219 CrossRef CAS PubMed.
- M. D. Joshi and J. L. Anderson, RSC Adv., 2012, 2, 5470–5484 RSC.
- L. Ramos, J. J. Ramos and U. A. Brinkman, Anal. Bioanal. Chem., 2005, 381, 119–140 CrossRef CAS PubMed.
- C. B. Ojeda and F. S. Rojas, Chromatographia, 2009, 69, 149–1159 Search PubMed.
- A. Jain and K. K. Verma, Anal. Chim. Acta, 2011, 706, 37–65 CrossRef CAS PubMed.
- A. A. Nuhu, C. Basheer and B. Saad, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2011, 879, 1180–1188 CrossRef CAS PubMed.
- S. Liu and P. K. Dasgupta, Anal. Chem., 1996, 68, 1817–1821 CrossRef PubMed.
- M. A. Jeannot and F. F. Cantwell, Anal. Chem., 1996, 68, 2236–2240 CrossRef CAS PubMed.
- K. Choi, J. Kim and D. S. Chung, Bioanalysis, 2011, 3, 799–815 CrossRef CAS PubMed.
- Z. A. ALOthman, M. Dawod, J. Kim and D. S. Chung, Anal. Chim. Acta, 2012, 739, 14–24 CrossRef CAS PubMed.
- E. Psillakis and N. Kalogerakis, Trends Anal. Chem., 2003, 22, 565–574 CrossRef CAS.
- E. Psillakis and N. Kalogerakis, Trends Anal. Chem., 2002, 21, 54–64 CrossRef.
- K. E. Rasmussen and S. P. Bjergaard, Trends Anal. Chem., 2004, 23, 1–10 CrossRef CAS.
- K. Shrivas and D. K. Patel, Spectrochim. Acta, Part A, 2011, 78, 253–257 CrossRef PubMed.
- P. M. Wiggins, Phys. A, 1997, 238, 113–128 CrossRef CAS.
- T. Arkawa, Arch. Biochem. Biophys., 1986, 248, 101–105 CrossRef.
- Y. B. Pakade and D. K. Tewary, J. Sep. Sci., 2010, 33, 3683–3691 CrossRef CAS PubMed.
- R. Sekar and H. F. Wu, Anal. Chem., 2006, 78, 6306–6313 CrossRef CAS PubMed.
- K. Shrivas and H. F. Wu, Rapid Commun. Mass Spectrom., 2007, 21, 3103–3108 CrossRef CAS PubMed.
- K. Shrivas and H. F. Wu, Anal. Chim. Acta, 2007, 605, 153–158 CrossRef CAS PubMed.
- K. Shrivas and H. F. Wu, J. Mass Spectrom., 2007, 42, 1637–1644 CrossRef CAS PubMed.
- H. F. Wu and C. H. Lin, Rapid Commun. Mass Spectrom., 2006, 20, 2511–2515 CrossRef CAS PubMed.
- X. Sun, Z. Miao, Z. Yuan, P. B. Harrington, J. Colla and H. Chen, Int. J. Mass Spectrom., 2011, 301, 102–108 CrossRef CAS PubMed.
- Y. Bai, J. Zhang, Y. Bai and H. Liu, Anal. Bioanal. Chem., 2012, 403, 2307–2314 CrossRef CAS PubMed.
- J. Xie, J. Yin, S. Sun, F. Xie, X. Zhang and Y. Guo, Anal. Chim. Acta, 2009, 638, 198–201 CrossRef CAS PubMed.
- H. F. Wu, S. K. Kailasa and C. H. Lin, Rapid Commun. Mass Spectrom., 2011, 25, 307–315 CrossRef CAS PubMed.
- P. Hu, Q. Ye and J. A. Loo, Anal. Chem., 1994, 66, 4190–4194 CrossRef CAS.
- S. Sun, Z. Cheng, J. Xie, J. Zhang, Y. Liao, H. Wang and Y. Guo, Rapid Commun. Mass Spectrom., 2005, 19, 1025–1030 CrossRef CAS PubMed.
- J. P. Xie, S. H. Sun, H. Y. Wang, Y. L. Zong, C. Nie and Y. L. Guo, Rapid Commun. Mass Spectrom., 2006, 20, 2573–2578 CrossRef CAS PubMed.
- H. Therisod, V. Labas and M. Caroff, Anal. Chem., 2001, 73, 3804–3807 CrossRef CAS.
- J. Thunig, L. Flo, S. Pedersen-Bjergaard, S. H. Hansen and C. Janfelt, Rapid Commun. Mass Spectrom., 2012, 26, 133–140 CrossRef CAS PubMed.
- P. Maienfisch, H. Huerlimann, A. Rindlisbacher, L. Gsell, H. Dettwiler, J. Haettenschwiler, E. Sieger and M. Walti, Pest Manage. Sci., 2001, 57, 165–176 CrossRef CAS.
- N. Campillo, P. Viñas, G. Férez-Melgarejo and M. Hernández-Córdoba, J. Agric. Food Chem., 2013, 61, 4799–4805 CrossRef CAS PubMed.
- H. F. Wu, H. Y. Ku and J. H. Yen, J. Sep. Sci., 2008, 31, 2288–2294 CrossRef CAS PubMed.
- L. Li, E. V. Romanova, S. S. Rubakhin, V. Alexeeva, K. R. Weiss, F. S. Vilim and J. V. Sweedler, Anal. Chem., 2000, 72, 3867–3874 CrossRef CAS.
- S. H. Yang, P. M. Reddy and Y. P. Ho, Anal. Chem., 2010, 82, 44–48 CrossRef CAS PubMed.
- J. L. Campbell and J. C. Le Blanc, Bioanalysis, 2011, 6, 645–657 CrossRef PubMed.
- Y. J. Kim and M. L. Doyle, Anal. Chem., 2010, 82, 7083–7089 CrossRef CAS PubMed.
- G. J. Van Berkel, A. D. Sanchez and J. M. Quirke, Anal. Chem., 2002, 74, 6216–6223 CrossRef CAS.
- G. J. Van Berkel, V. Kertesz, K. A. Koeplinger, M. Vavrek and A. N. Kong, J. Mass Spectrom., 2008, 43, 500–508 CrossRef CAS PubMed.
- G. J. Van Berkel and V. Kertesz, Anal. Chem., 2009, 81, 9146–9152 CrossRef CAS PubMed.
- G. J. Van Berkel, V. Kertesz and R. C. King, Anal. Chem., 2009, 81, 7096–7101 CrossRef CAS PubMed.
- V. Kertesz and G. J. Van Berkel, Anal. Chem., 2010, 82, 5917–5921 CrossRef CAS PubMed.
- V. Kertesz and G. J. Van Berkel, J. Mass Spectrom., 2010, 45, 252–260 CrossRef CAS PubMed.
- G. J. Van Berkel and V. Kertesz, Rapid Commun. Mass Spectrom., 2013, 27, 1329–1334 CrossRef CAS PubMed.
- R. A. Craven and R. E. Banks, Proteomics, 2001, 1, 1200–1204 CrossRef CAS.
- J. Quanico, J. Franck, C. Dauly, K. Strupat, J. Dupuy, R. Day, M. Salzet, I. Fournier and M. Wisztorski, J. Proteomics, 2013, 79, 200–218 CrossRef CAS PubMed.
- C. K. Chiang, W. T. Chen and H. T. Chang, Chem. Soc. Rev., 2011, 40, 1269–1281 RSC.
- Z. J. Zhu, V. M. Rotello and R. W. Vachet, Analyst, 2009, 134, 2183–2188 RSC.
- S. K. Kailasa and H. F. Wu, Analyst, 2012, 137, 1629–1638 RSC.
- S. K. Kailasa and H. F. Wu, J. Proteomics, 2012, 75, 2924–2933 CrossRef CAS PubMed.
- C. K. Chiang, Y. W. Lin, W. T. Chen and H. T. Chang, Nanomedicine, 2010, 6, 530–537 CrossRef CAS PubMed.
- C. H. Teng, K. C. Ho, Y. S. Lin and Y. C. Chen, Anal. Chem., 2004, 76, 4337–4342 CrossRef CAS PubMed.
- S. K. Kailasa, K. Kiran and H. F. Wu, Anal. Chem., 2008, 80, 9681–9688 CrossRef CAS PubMed.
- S. K. Kailasa and H. F. Wu, Analyst, 2010, 135, 1115–1123 RSC.
- K. Shrivas, S. K. Kailasa and H. F. Wu, Proteomics, 2009, 9, 2656–2667 CrossRef CAS PubMed.
- Y. Ke, S. K. Kailasa, H. F. Wu and Z. Y. Chen, Talanta, 2010, 83, 178–184 CrossRef CAS PubMed.
- S. K. Kailasa and H. F. Wu, Rapid Commun. Mass Spectrom., 2011, 25, 271–280 CrossRef CAS PubMed.
- K. Shrivas, S. K. Kailasa and H. F. Wu, Rapid Commun. Mass Spectrom., 2009, 23, 3603–3607 CrossRef CAS PubMed.
- H. F. Wu and F. T. Chung, Rapid Commun. Mass Spectrom., 2011, 25, 1779–1786 CrossRef CAS PubMed.
- L. Shastri, S. K. Kailasa and H. F. Wu, Rapid Commun. Mass Spectrom., 2009, 23, 2247–2252 CrossRef CAS PubMed.
- P. R. Sudhir, H. F. Wu and Z. C. Zhou, Anal. Chem., 2005, 77, 7380–7385 CrossRef CAS PubMed.
- L. Shastri, S. K. Kailasa and H. F. Wu, Talanta, 2010, 81, 1176–1182 CrossRef CAS PubMed.
- P. R. Sudhir, K. Shrivas, Z. C. Zhou and H. F. Wu, Rapid Commun. Mass Spectrom., 2008, 22, 3076–3086 CrossRef CAS PubMed.
- F. Ahmad and H. F. Wu, Analyst, 2011, 136, 4020–4027 RSC.
- K. Shrivas and H. F. Wu, Anal. Chem., 2008, 80, 2583–2589 CrossRef CAS PubMed.
- S. K. Kailasa and H. F. Wu, Talanta, 2010, 83, 527–534 CrossRef CAS PubMed.
- A. R. Bhat and H. F. Wu, Rapid Commun. Mass Spectrom., 2010, 24, 3547–3552 CrossRef CAS PubMed.
- M. Manikandan, N. Hasan and H. F. Wu, Talanta, 2013, 107, 167–175 CrossRef CAS PubMed.
- K. Y. Chen, T. C. Yang and S. Y. Chang, J. Am. Soc. Mass Spectrom., 2012, 23, 1157–1160 CrossRef CAS PubMed.
- K. Shrivas and H. F. Wu, Analyst, 2012, 137, 890–895 RSC.
- S. K. Kailasa and H. F. Wu, Analyst, 2012, 137, 4490–4496 RSC.
- S. K. Kailasa and H. F. Wu, Talanta, 2013, 114, 283–290 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |