Differential modulation of lactim–lactam tautomerism process of an isoindole fused imidazole system in three different micellar assemblies of varying surface charge: a spectroscopic approach to various photophysical properties†
Debarati Ray,
Animesh Pramanik and
Nikhil Guchhait*
Department of Chemistry, University of Calcutta, 92 A. P. C. Road, Calcutta-700009, India. E-mail: nguchhait@yahoo.com; Fax: +91-33-2351-9755; Tel: +91-33-2350-8386
First published on 27th February 2014
Abstract
This article describes a detailed characterization of the binding interaction of a pharmaceutically important isoindole fused imidazole derivative, namely 1-(2-hydroxy-5-methyl-phenyl)-3,5-dioxo-1H-imidazo-[3,4-b] isoindole (ADII) with biomimetic micellar assemblies of varying surface charge using steady state absorption, emission and time resolved emission spectroscopy. The studied molecule ADII is known to exhibit an intramolecular proton transfer process through a four member hydrogen bonding system resulting in a lactim lactam isomerisation process. Upon interaction with the micellar environment, a significant change in the absorption and emission profiles of ADII is observed due to differential modulation of inherent proton transfer in the three studied ionic and non-ionic micellar environments. The micropolarity measurement and fluorescence quenching experiments have been performed to evaluate the binding location of the probe (ADII) in the three micellar assemblies. The differential binding affinity of the probe with the micelles also results in different rotational relaxational motion of the probe within the encapsulated microheterogeneous environments.
1. Introduction
In the past two decades, it has been well recognized that surfactants play a crucial role in both fundamental and applied science.1 One important property of a surfactant is the formation of colloidal sized clusters in aqueous medium, known as micelles which are thermodynamically very stable and formed above a critical concentration of surfactant, known as the critical micellar concentration (CMC). One major problem in medicinal chemistry is the poor solubility of sparingly soluble drugs in aqueous medium which is a major obstacle in the development of therapeutic agents. This problem can be overcome by micelles as they are able to enhance the aqueous solubility of poorly-water soluble drugs hence increase the bioavailability of the drugs.2 In addition to improving solubility and bioavailability of drugs, micelles are also used as drug carriers in numerous drug delivery and drug targeting systems.3,4 Moreover, micellar systems are also regarded as simple models of biomembranes as the inherent compositional and functional complexities associated with the true biological membranes is completely absent in micellar medium.5,6 Hence study of the binding interaction of numerous fluorophores with micelles is highly significant.In fundamental science, the photophysical/photochemical processes of the micelle entrapped fluorophores are noticeably modified due to their location and nature of surrounding environment (within micelle) with respect to the bulk aqueous phase.7–14 A number of recent studies have reported the influence of micelles, reverse micelles, and cyclodextrins on the intramolecular charge transfer processes but the study of reactivity and dynamics of photo-induced inter or intramolecular proton transfer processes in micelle and reverse micelle are comparatively less. The intermolecular proton transfer processes are also found to be markedly affected in micellar medium.8
As per our knowledge goes to the modification of intramolecular proton transfer process of a four member hydrogen bonding system in micellar medium is rare. In our recent work we have reported a detail photophysical characterization of a heterocycle containing isoindole fused imidazole moiety with a phenolic subunit namely 1-(2-hydroxy-5-methyl-phenyl)-3,5-dioxo-1H-imidazo-[3,4-b] isoindole (ADII) which exhibits lactim–lactam isomerisation through the operation of intramolecular proton transfer mechanism across the four member intramolecular hydrogen bonding network. ADII yields dual emission corresponding to lactim and lactam emission in all organic solvents.15 Both steady state and time resolved fluorescence measurements suggest that ESIPT process in ADII is less favorable in water than in other organic solvents. Therefore its photophysical behavior is expected to be interesting in the various microheterogeneous environments formed by the self-aggregation of the different types of surfactant molecules. The present work deals with the modification of inherent excited state proton transfer behavior of ADII in three different micellar mediums formed by surfactants like cetyltrimethylammonium bromide (CTAB), Triton X-100 (TX-100), and sodium dodecyl sulfate (SDS). Moreover, the title compound ADII is widely used as templates to design various biologically active agents in the medicinal chemistry.16–19 Therefore, the solubility, binding affinity and the location of probe binding site of such biologically potent molecule in the three micellar environments should be significant and interesting for the further understanding of its interaction with relevant biological targets.
2. Experimental
2.1. Materials
The title compound 1-(2-hydroxy-5-methyl-phenyl)-3,5-dioxo-1H-imidazo-[3,4-b] isoindole (ADII) was synthesized as previously reported.15,20 The surfactants sodium dodecyl sulfate (SDS), cetyltrimethylammonium bromide (CTAB) and Triton X-100 (TX-100) have been purchased from Sigma Chemical Co. and were used without further purification. Tris buffer and hydrochloric acid (HCl) from E-Merck were used to prepare the Tris–HCl buffer (pH 7.4) in deionized water from Milli-Q water purification system (Millipore). The solvent 1,4-dioxane is of UV spectroscopy grade (Spectrochem, India).Copper(II) perchlorate (Cu(ClO4)2·H2O) from Sigma-Aldrich, USA and cetyl pyridinium chloride (CPCL) from Loba Chemie were used for fluorescence quenching studies.
2.2. Instrumentation and methods
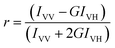 | (1) |
 | (2) |
 | (3) |
 . The quality of the fits has been judged from χ2 criterion and visual inspection of the residuals of the fitted functions to the actual data.
. The quality of the fits has been judged from χ2 criterion and visual inspection of the residuals of the fitted functions to the actual data.For time-resolved anisotropy measurements, the fluorescence decay curves were recorded at vertical and horizontal positions of the polarizer and analyzed by the following equations:
where, I‖ and I⊥ are the intensities collected at emission polarizations parallel and perpendicular, respectively, to the polarization axis of the excitation beam. I(t) and r(t) are the time-dependent intensity (at the magic angle) and anisotropy decay, respectively. Thus, the anisotropy decay function r(t) has been constructed from these I‖(t) and I⊥(t) decays:21
 | (4) |
3. Results and discussions
3.1. Steady-state absorption study
The detailed photophysical characterization of ADII has been reported by our group recently.15 The absorption spectrum of ADII in water was characterized by two absorption band at ∼310 nm and ∼400 nm for the lactim and the lactam form, respectively. These two absorption bands are practically independent of the nature of the organic solvents but they are interestingly modified in presence of varying concentrations of acid and base in aqueous or non-aqueous (acetonitrilic) medium. In order to examine the photophysics of ADII in the micellar microenvironment, the UV-vis spectra of the molecule have been investigated systematically in the cationic, anionic, and nonionic micelles environment. Fig. 1 displays the absorption profile of ADII in aqueous buffer medium with varying concentrations of anionic surfactant SDS, cationic surfactant CTAB and non-ionic surfactant TX-100. In SDS micelle, intensity of the lactam band is found to be gradually enhanced whereas intensity of the lactim band intensity is slightly increased. On the other hand, in non-ionic surfactant, intensity of the lactam band increases with broadening of the absorption band. Monitoring of lactim absorption band in presence of TX-100 is not possible because of overlapping region of TX-100 and the lactim band of the probe. The increase of both the absorption bands of ADII in SDS medium suggest the increasing solubility of the probe in the aqueous phase but the more increase of lactam absorption band in presence of anionic surfactant medium may also indicate that the ground state prototropic conversion is favored towards the lactam form in SDS medium. More interesting results are observed when the molecule ADII is placed in the cationic surfactant medium. Fig. 1b reveals that with increasing concentration of CTAB, a new band gradually generates at 480 nm. By comparing this result with the absorption spectrum obtained during addition of triethylamine in acetonitrilic solution of ADII (vide inset of Fig. 1b), it is clear that the new band appeared in the CTAB medium is the anionic species generated from the lactam form (Chart 1).15 There are reports regarding the higher basicity of CTAB micellar interface due to the lower counter ion concentration near the interface.22,23 Therefore, absorption spectral study reveals that the solubility of ADII molecules is enhanced in anionic and neutral micelles with shifting of prototropic equilibrium towards the lactam form and more importantly the neutral ADII molecule becomes anionic in CTAB medium. | ||
| Chart 1 Schematic representation of lactim and lactam tautomerisation of ADII compound and the possible anionic lactam form. | ||
3.2. Steady-state emission study
We have earlier reported that ADII shows dual emission in all organic solvents, where the high energy emission was assigned to the emission from the lactim isomer and the low energy emission due to the lactam form.15 The fluorescence spectra of ADII in different micellar systems (here SDS, CTAB and TX-100) with varying concentrations of surfactants have been recorded at the excitation wavelength of 320 nm (lactim excitation) and 400 nm (lactam excitation) and are presented in Fig. 2. Like water, in Tris aqueous buffer also the emission intensity of the lactim form of ADII is observed to be much higher than the lactam emission. As displayed in Fig. 2a, lactam emission of ADII drastically enhances in presence of anionic surfactant SDS whereas, the emission intensity of the lactim form is nominally increased in SDS medium. In neutral surfactant TX-100, the lactam emission remains practically unchanged up to a certain concentration range of TX-100, but at higher concentration it enhances largely. Like SDS medium, the lactim emission intensity slightly increases in TX-100 medium (Fig. 2c). It is also to note that in presence of both SDS and TX-100 medium, the lactam emission maxima shift from 513 nm (in aqueous buffer) to 508 nm (in 7 mM SDS) and 501 nm (in 0.3 mM TX-100), i.e., a blue shift is observed. We have earlier seen that emission maximum of the lactam form is shifted to blue in the medium of lower polarity whereas, the lactim emission maximum is practically invariant of the polarity of the medium.15 Therefore, the observed blue shifts of the lactam emission maxima in the anionic and neutral micellar medium (SDS and TX-100) definitely suggest that the molecule must move from the more polar aqueous environment to a less polar, more hydrophobic micellar environment and the hydrophobicity around the probe is higher in case of TX-100 medium. Another interesting result is obtained from the emission results of ADII in SDS and TX-100 medium at excitation wavelength 320 nm. Fig. 3 represents the variation of relative emission intensity (both for lactim and lactam emission), I/I0, (where, I and I0 are the emission intensities of the probe molecule in the presence and absence of surfactants, respectively) against the concentration of surfactants (here, SDS and TX-100). Upon increasing concentration of SDS, the slope of the plot is much higher in case of lactam emission than lactim emission. On the other hand, in presence of TX-100 the variation of emission intensity plot is steeper in case of lactim emission up to a certain concentration of TX-100 (i.e., upto CMC of TX-100) after that a sharp increase of I/I0 is observed for lactam emission. These results signify that anionic surfactant medium (SDS) accelerates the excited state prototropic equilibrium towards lactam isomer of ADII whereas, in TX-100 medium lactim form is predominant upto the CMC of TX-100. However, more dire modification on the emission profile is observed when cationic surfactant CTAB is added to the aqueous buffer solution of ADII (vide Fig. 2b). In presence of CTAB, the lactim emission dramatically increases whereas the lactam emission quenches up to a certain concentration of CTAB and then again rises at higher concentration of CTAB. This result is only explainable by considering the results obtained in the steady state absorption study. From absorption study it is clear that the molecule ADII becomes anionic (more specifically the lactam form) in CTAB medium. Therefore, the quenching of lactam emission in CTAB medium (upto a certain concentration) suggests that the ESIPT phenomenon is hampered due to the deprotonation of lactam form (vide Chart 1) in the said medium. It is also mentionable that at higher concentration of organic base (TEA) results the complete diminish of the lactam emission but here at higher concentration of CTAB the lactam emission again raises. This is probably due to the stability gained by the anionic lactam form in cationic micellar medium results the increment of emission intensity. Again a blue shift in the emission maxima (513 nm in aqueous buffer medium to 481 nm in 2 mM CTAB medium) is observed as the anionic lactam form experiences a more hydrophobic environment in CTAB medium than the bulk aqueous phase. | ||
| Fig. 3 Variation of lactim and lactam emission intensity of ADII at λex = 320 nm in (a) SDS and (b) TX-100 medium. | ||
It is observed that the excited state behavior of ADII is dependent of the concentrations of the surfactants. More specifically, differential behavior of lactam emission intensity is observed at low and high concentration of surfactants. Therefore we have plotted the variation of emission intensity of the ADII as a function of surfactant concentration which follows a specific pattern. A close inspection of Fig. S1† reveals a break point in the pattern of variation of fluorescence intensity in each plot and the concentrations corresponding to the break points are found to be in good harmony with the literature value24–27 of the CMC of the corresponding surfactants and the estimated CMC values obtained from the figure are presented in Table 1.
| Environment | CMC (mM) | CMC (mM) (literature report) | K × 105 (M−1) | ΔG (kJ mol−1) | KSV (M−1) for Cu2+ | KSV (mM−1) for CpCl |
|---|---|---|---|---|---|---|
| Aqueous buffer | — | — | — | — | 141.83 | — |
| SDS | 4.06 | 4.20 | 4.12 | −32.24 | 213.15 | 0.2 |
| CTAB | 0.205 | 0.41 | 5.97 | −33.16 | 3.45 | 15.44 |
| TX-100 | 0.104 | 0.13 | 9.98 | −34.44 | 111 | 14.80 |
3.3. Determination of ADII–micelle binding constant
The interaction and binding of a small molecule with different micelles depend on the nature of the molecules and also on the microenvironment of the micelles (Table 2).28 In order to obtain a quantitative estimation of the binding interaction of ADII with different micellar systems and the feasibility of the process, the binding constants (K) and the free energy changes (ΔG) for the processes have been determined using the very well established method described by Almgren et al.29| Environment | τ1 (ns) | τ2 (ns) | τ3 (ns) | α1 | α2 | α3 | χ2 | 〈τ〉 (ns) |
|---|---|---|---|---|---|---|---|---|
| SDS (mM) | ||||||||
| 0 | 1.93 | 6.13 | 0.151 | 0.13 | 0.07 | 0.80 | 1.04 | 3.912 |
| 3 | 1.70 | 6.06 | 0.225 | 0.26 | 0.10 | 0.64 | 1.02 | 3.738 |
| 4 | 1.72 | 6.18 | 0.228 | 0.25 | 0.10 | 0.65 | 1.06 | 3.839 |
| 5 | 1.67 | 6.20 | 0.221 | 0.25 | 0.10 | 0.65 | 1.04 | 3.871 |
| 8 | 1.70 | 6.14 | 0.246 | 0.26 | 0.10 | 0.64 | 0.97 | 3.757 |
| CTAB (mM) | ||||||||
| 0 | 1.93 | 6.13 | 0.151 | 0.13 | 0.07 | 0.80 | 1.04 | 3.912 |
| 0.2 | 1.80 | 6.10 | 0.145 | 0.10 | 0.06 | 0.84 | 1.05 | 3.854 |
| 0.4 | 1.58 | 6.14 | 0.196 | 0.16 | 0.05 | 0.79 | 1.05 | 3.239 |
| 0.6 | 1.76 | 6.84 | 0.225 | 0.22 | 0.09 | 0.70 | 1.09 | 4.246 |
| 0.8 | 1.78 | 7.20 | 0.214 | 0.25 | 0.11 | 0.64 | 1.17 | 4.748 |
| 1.0 | 1.85 | 7.65 | 0.229 | 0.28 | 0.12 | 0.60 | 1.005 | 5.092 |
| 1.2 | 1.98 | 8.37 | 0.257 | 0.29 | 0.20 | 0.51 | 1.09 | 6.381 |
| TX-100 (mM) | ||||||||
| 0.00 | 1.93 | 6.13 | 0.151 | 0.13 | 0.07 | 0.80 | 1.04 | 3.912 |
| 0.16 | 1.78 | 5.86 | 0.211 | 0.27 | 0.09 | 0.64 | 1.00 | 3.477 |
| 0.20 | 1.70 | 5.75 | 0.218 | 0.28 | 0.09 | 0.63 | 1.04 | 3.380 |
| 0.25 | 1.62 | 5.48 | 0.195 | 0.26 | 0.09 | 0.65 | 1.00 | 3.275 |
| 0.30 | 1.63 | 5.53 | 0.211 | 0.27 | 0.09 | 0.64 | 1.02 | 3.260 |
Fig. S2† shows the linear plots of (I∞ − I0)/(It − I0) vs. 1/[M] for SDS, CTAB, and TX-100 micellar systems. The binding constants (K) have been calculated from the slope and the values are given in Table 1. The free energy changes (ΔG) due to the binding interaction have been calculated from the obtained binding constant values using the relationship ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K and are presented in Table 1. The negative free energy change indicates that with all micellar environments the binding process is spontaneous. From the estimated binding constant values, it has been observed that the binding of ADII with the nonionic surfactant (TX-100) is the highest and lowest for anionic surfactant SDS while for cationic surfactant the binding strength is intermediate.
K and are presented in Table 1. The negative free energy change indicates that with all micellar environments the binding process is spontaneous. From the estimated binding constant values, it has been observed that the binding of ADII with the nonionic surfactant (TX-100) is the highest and lowest for anionic surfactant SDS while for cationic surfactant the binding strength is intermediate.
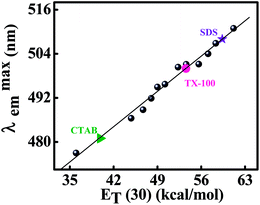
3.4. Polarity of the micellar microenvironment: estimation of the probable location of the probe
Micelles are known to have microheterogeneous environments therefore it is an important task to determine the microscopic polarity of the drug binding site in it. Detection technique using fluorescent probe is a favorable one as the fluorescence spectroscopy is much simpler and precise technique.21,27,30,31 The local polarity of the probe binding site in the different micellar medium can be estimated by comparing the spectral characteristics (especially the emission maxima) in those medium with those in pure solvents or solvent mixture of known polarity.21,27,30,31 In an earlier study, it was observed that the lactam emission of ADII is dependent on the polarity of the medium.15 Here we have monitored the fluorescent behavior of ADII in different composition of water–dioxane mixture of known polarity and is presented in Fig. S3.† It is clear from the figure that with decreasing the polarity of the medium a clear blue shift of lactam emission is observed. A calibration curve has been constructed by plotting the lactam emission maxima of ADII in different water–dioxane mixture with the empirical solvent polarity parameter, ET(30) of the corresponding medium (vide Fig. 4). In the previous section, we have seen the modulation of emission maxima of ADII in different micellar environment. Putting the values of PT maxima of ADII bound to the micelles of different surfactants on the calibration line to determine the micropolarities around the probe in the micellar medium and the estimated ET(30) values are 59.37, 39.84 and 53.56 kcal mol−1 in SDS, CTAB and TX-100, respectively. The estimated values of micropolarity of the ADII in the immediate vicinity of its interaction site within the micellar environments indicates the probe must be resided on the lower polarity region in comparison to the aqueous buffer phase (ET(30) = 63.1 kcal mol−1). Typically, a charged micellar structure is characterized by different distinct layers: (a) nonpolar core containing the hydrocarbon tails of the surfactant; (b) compact head group region (Stern layer) and (c) relatively wider Gouy–Chapmann region containing the counter ions followed by the aqueous bulk phase at an infinite distance from the core.32 On the other hand a neutral micellar structure is characterized by two distinct layers: (a) a nonpolar core and (b) the palisade layer. The palisade layer is composed of oxyethylene unit (head group) of the surfactant molecule and a large no of water molecule orient around these head groups. The micellar core of neutral surfactant is highly nonpolar (similar to the hydrocarbon solvents) and the palisade layer is quite polar because of the presence of large water molecule.33 Recently, Kumbhakar et al. determined the micropolarity around coumarin 153 in TX-100 and TX-165 micelles in the Lippert–Mataga scale.33 They suggested that the fluorophore resides in the palisade region of both of the micelles where the dielectric constant (ε) values for the localization sites of the dye in TX-100 and TX-165 micelles were 22.4 and 26.7, respectively. In our present study, the estimated ET(30) value for the binding site of ADII in TX-100 is 53.56 kcal mol−1 which falls at intermediate region of pure ethanol (ET(30) = 51.9 kcal mol−1 and ε = 24.55) and methanol (ET(30) = 55.5 kcal mol−1 and ε = 32.7) solvent. Therefore, the dielectric constant (ε) of the binding site of ADII in TX-100 medium should fall within 24.55 to 32.7. Hence, comparing the results obtained by Kumbhakar et al. we can conclude that our probe ADII must be localized in the palisade layer of neutral micellar environment. Whereas, on comparing the polarities of the Stern layer of the micellar systems as determined earlier using betaine dye,34 4-N,N′-dimethylamino-3-hydroxyflavone35 and 1-anilino-8-naphthalene sulfonate36 another fluorescent probe,27,37,38 we can infer that the probe must be resided on the Stern layer of the both cationic and anionic micelle. Moreover, the estimated ET(30) value of the site of the bound probe in anionic micelle is found to be of 59.37 kcal mol−1 which is comparatively more close to the aqueous bulk phase (ET(30) = 63.1 kcal mol−1) therefore it is quite justified that though the molecule resides on the stern layer of the anionic micelle but it is more exposed to the wider Gouy–Chapmann region. Interestingly, the ET(30) value of the probe binding site within CTAB micellar medium (ET(30) = 39.84) is close to the value the pure acetonitrile (ET(30) = 46) medium. Therefore our interpretation regarding the behavior of ADII in cationic surfactant CTAB based on the result obtained in triethylamine (an organic base) treatment in acetonitrilic medium is thus justified.3.5. Fluorescence quenching
Recently, the metal ion Cu2+ has been used extensively for studying the fluorescence quenching of a probe molecule bound in different supramolecular assemblies with an aim to see the degree of accessibility of the fluorophore in different surfactant media or supramolecular assemblies toward the quencher.21,27,28 The thought behind this study is to get an idea about the location of the probe molecule as the ionic quencher Cu2+ is not supposed to be available in the micellar core due to the very low polarity of the said region but it is expected to be available in aqueous phase as well as in the micelle–water interface.21,27,28 It is also mentionable that the nature of surfactant also plays an important role for the accessibility of Cu2+ in micellar environments i.e., the charge of the head group of micelles also dictates the accessibility of the bound fluorophore to the ionic quencher. The quenching rate constants for the different systems were determined using the Stern–Volmer relation where the terms have their usual meanings:21where, I0 is the original fluorescence intensity, I is the quenched intensity of the fluorophore (ADII), [Q] is the molar concentration of the quencher and KSV is the Stern–Volmer quenching constant. The higher is the magnitude of KSV, the better is the quenching process suggests the greater is the degree of exposure of the quencher to the probe.21,27,28,38,39 The Stern–Volmer plots for Cu2+ ion-induced quenching of ADII in aqueous buffer and in various micellar environments are displayed in Fig. 5 and the corresponding KSV values are presented in Table 1. From this result it is found that the quenching efficiency is the highest in case of anionic SDS micelle (KSV in SDS micelle is greater than that in aqueous medium also) and minimum for cationic CTAB micelle whereas for neutral TX-100 micelle it is intermediate. As we have earlier described that the charge of the head group of micelles also dictates the accessibility of the bound fluorophore to the ionic quencher28 therefore the very high KSV value in SDS medium and the least KSV value in CTAB medium is justified, as in case of anionic micelle strong electrostatic attractive interaction is operative whereas for cationic micelle it is the repulsive interaction. For further verification of the locality of bound probe in the micellar medium we have chosen a quencher CPCl which mixes ideally with the surfactants and may enter into the Stern layer thereby causing fluorescence quenching.38,40,41 Moreover, quencher with properties similar to the surfactant studied offers important advantages over the more conventional quenchers like iodide, bromide and Cu2+ etc. Fig. S4† depicts the Stern–Volmer plots for the quenching of ADII fluorescence in all the micellar environments using CPCl as quencher and the obtained KSV values for the quenching process is presented in Table 1. The obtained KSV values follow the order: SDS < TX-100 < CTAB. The least KSV value obtained in SDS medium using CPCl as a quencher is a little confusing as from the estimated location of the probe through micropolarity measurement suggests that the probe resides mostly at the Gouy–Chapmann region of SDS micelle which is the outermost region of the corresponding micelle. This result is in good agreement with the other probe–SDS complex where the probe was also found to be resided at the outermost region of SDS micelle.38,40 This is probably explained by the fact that as ADII resides at the Gouy–Chapmann region of SDS micelle which consists of positively charged counter ion (as the head group of SDS is anionic) therefore, the cetyl pyrimidinium cation does not come in contact with the bound ADII in SDS medium due to the strong repulsion of the positive charge present in the Gouy–Chapmann region of the corresponding micellar medium.
 | ||
| Fig. 5 Stern–Volmer plots for the fluorescence quenching of ADII by Cu2+ ions in aqueous buffer and different micellar (SDS, CTAB and TX-100) medium. | ||
3.6. REES
According to Kasha's rule, the fluorescence spectra of a fluorophore is independent of excitation wavelength but that is a good assumption for fluorophores in bulk non viscous solvents where the dipolar relaxation of the solvent molecules around the excited fluorophore is much faster than the fluorescence lifetime.21 However, if the dipolar relaxation of the solvent molecules around the excited fluorophore is slow such that the relaxation time is found to be comparable to or longer than the fluorescence lifetime of the fluorophore then the molecule can exhibit the excited state wavelength dependent emissive behavior.21,42–45 Then the observed red shift of the emission wavelength maximum caused by the shift in the excitation wavelength toward the red edge of the absorption band is termed as the red edge excitation shift (REES). Generally, this effect is observed when a polar fluorophores is bound to a restricted media such as protein, membrane and micelle etc. where the mobility of the fluorophore is considerably reduced.44 But the observation of REES phenomenon of fluorophores in restricted media is not familiar to literature. Thus, it is of our interest to investigate whether the probe ADII in different micellar medium is capable of showing REES phenomenon or not.It is evident from Fig. 6 that on shifting the excitation wavelength from 410 nm to 450 nm results the emission maxima of different micelles (SDS, CTAB and TX-100) bound ADII shifts to the red end. This observation indicates that the micelle bound fluorophore is in an environment where its mobility is restricted. It is also interesting to mention that a high degree of REES is observed in the cationic micellar medium which is presented in Fig. S5† and the observation is interpreted as follows. As in cationic micellar medium ADII becomes a charged species therefore the electrostatic interaction is expected to dominate over other non-electrostatic one. This interaction plays the crucial role in distribution of energetically different molecules in the ground state and this is the most important condition which must be satisfied to observe REES42,43,45 phenomenon.
3.7. Fluorescence decay study
By virtue of its sensitivity to the environments and various excited state affairs, fluorescence lifetime serves as a faithful indicator to explore various photochemical/photophysical processes of a fluorophore in the different microheterogeneous medium.21,27,28,39 This technique has been widely used to develop an understanding of the interaction between the probe and the micelles.21,27,28,39 Therefore to determine how the fluorescence lifetime of ADII is affected in various micellar assemblies, we have studied the fluorescence decay measurements in the three studied micellar medium. The typical time-resolved fluorescence decay profiles of ADII in various concentrations of surfactant medium are displayed in Fig. S6† at excitation wavelength 336 nm. The collected data summarized in Table 2 suggests that the individual time constant values (τi) of different species and their relative amplitude values (αi) are interestingly modified in the studied micellar medium. In aqueous buffer medium ADII shows triexponential decay function for both excitation at 336 nm (lactim excitation) and 450 nm (lactam excitation) where the open conformer of lactim form is dominating and the other two (closed form and lactam form) are of minor contributing.15 Upon interaction with the micellar environment (at both excitation wavelengths) the time resolved fluorescence spectra of ADII remains as triexponential function but the relative amplitude of the three components are interestingly modified. As seen in Table 2, the TCSPC results of ADII in anionic and neutral surfactant medium are found to be very similar with each other with a little dissimilarity in the average lifetime values. In SDS medium, at λex = 336 nm and λmonem = 430 nm (monitoring the lactim emission but this emission maxima shares an overlapping zone with the lactam emission), the individual lifetime of the open conformer of lactim form slightly increases whereas that of for the closed conformer and lactam form is practically unchanged. On the contrary, the relative amplitude corresponding to the open form of lactim isomer is found to be decreased from 80% (in aqueous buffer) to 64% in 8 mM SDS but it increases for the closed conformer and lactam form (for closed conformer and lactam form, α increases from 13% (in aqueous buffer) to 26% (in 8 mM SDS) and 7% (in aqueous buffer) to 10% (in 8 mM SDS), respectively). Therefore, in summary the total relative amplitude of lactim isomer (both open and closed form) decreases from 93% (in aqueous buffer) to 90% (in 8 mM SDS and 91% in 0.3 mM TX-100 medium) and that of for lactam emission it rises from 7% (in aqueous buffer) to 10% (in 8 mM SDS and 9% in 0.3 mM TX-100 medium). These results again justified that at higher concentration of SDS and TX-100, the excited state prototropic equilibrium is accelerated towards lactam form. In presence of cationic micelle, at λex = 336 nm and λem = 430 nm, the individual lifetime of the lactam form noticeably increases from 6.13 ns (in aqueous buffer) to 8.37 ns (in 1.2 mM CTAB) and its relative population also enhances from 7% (in aqueous buffer) to 20% (in 1.2 mM CTAB). Conversely the relative decrease and increase in the amplitude of the open and the closed form of lactim isomer are also noticeable. This behavior of ADII in CTAB medium is only explainable on the ground of its steady state spectral (absorption and emission) modification in CTAB medium. From steady state absorption and emission study it is clear that the molecule ADII is no longer be a neutral one in CTAB medium, it becomes anionic species as the lactam form of ADII gets deprotonated. Like TCSPC measurement, during steady state emission study the lactam emission of ADII decreases up to CMC value of CTAB and after CMC value it again rises due to the stabilization of the anionic form in cationic micellar medium. Therefore the same argument is also applicable to justify the increase of individual lifetime of lactam form at higher concentration (after CMC) of CTAB.| Environment | β (%) | θ (ns) | χ2 |
|---|---|---|---|
| Aqueous buffer | 100 | 0.183 | 1.04 |
| SDS | 100 | 1.05 | 1.05 |
| CTAB | 100 | 1.33 | 1.05 |
| TX-100 | 100 | 3.35 | 1.04 |
Another interesting result is obtained during TCSPC measurement of ADII in CTAB medium. The time-resolved fluorescence decay properties of bound ADII in the cationic micellar environment have been found to be sensitive to the monitoring emission wavelength.21,44 Fig. 7 displays the change in mean lifetime of ADII as a function of monitoring emission wavelength when the excitation wavelength is fixed at 450 nm. Upon shifting the emission wavelength gradually to the red end of the spectrum (from λem = 510 nm to 540 nm with 10 nm increment), the mean fluorescence lifetime of the probe is found to increase progressively (in 1.5 mM CTAB medium τav increases from 2.76 ns (at monitoring wavelength 510 nm) to 3.810 ns at λmonem = 540 nm). Such enhancement of average lifetimes at the red-edge of emission band of the micelle-encapsulated fluorophore may be interpreted as follows. Because during TCSPC measurement when the monitoring wavelength is set at the red-edge of emission band, predominantly the decay form the solvent relaxed fluorophores is observed.21,44 As the shorter wavelengths of emission select predominantly for unrelaxed or/and partially relaxed fluorophores which decay more rapidly as the solvent relaxed fluorophore. In other two micellar system this observation is not observed. The increase of mean fluorescence lifetime at the red-edge of emission band is only observed in CTAB medium and may be explained as follows. During REES measurement, maximum REES is observed in CTAB medium which is probably due to the formation of ionic species (detail discussion regarding REES measurement is mentioned previously). During TCSPC measurement at varying emission wavelength it is found that the individual time constant of lactam form of ADII is noticeably increased when monitoring wavelength is set at 540 nm hence the average lifetime increases, whereas in other two micellar environment no such observation is found out.
3.8. Time resolved anisotropy
The time resolved fluorescence anisotropy is also a sensitive technique to understand the microenvironment of the surrounding of the fluorophore which in turn influences the rotational motion and/or rotational-relaxation of the probe molecule in the studied organized medium.21,27,28,39,46,47 Fig. 8 reveals the typical time resolved fluorescence anisotropic decay profile of ADII in the studied micellar medium and the corresponding decay parameters are tabulated in Table 3. The anisotropic decay is described by the following expression:21,27,28,39,46,47where, βi denotes the preexponential factor for the i-th rotational correlation time θi.
 | ||
| Fig. 8 Typical time-resolved fluorescence anisotropy decay profile of ADII in aqueous buffer, 6.0 mM SDS, 1.00 mM CTAB, and 0.26 mM TX-100. | ||
In pure aqueous buffer medium, the anisotropic decay is found to be single exponential with rotational relaxation time of 183 ps. In three different micellar medium the time resolved anisotropic decays are also well fitted to the single exponential function, but the rotational relaxation time of ADII is noticeably enhanced. This result confirms that in micellar medium the probe molecule is definitely motionally restricted. It is also mentionable from the data presented in table that the rotational relaxation time of ADII in TX-100 is the slowest and comparatively fastest in anionic SDS medium whereas, in CTAB it is intermediate. This is because of the more compactness and rigidity of the TX-100 micellar environment which limits the rotational motion of the fluorophore.
4. Conclusion
The present work demonstrates the photophysical behavior of a biologically important hetero cyclic compound ADII in three different micellar assemblies of varying surface charge. The spectroscopic results reveal the differential behavior of ADII in the cationic micellar environment, CTAB than the other two micellar assemblies, SDS and TX-100. Most importantly, in CTAB medium the neutral molecule ADII becomes anionic due to the deprotonation of lactam form in micelle water interfacial region hence the ESIPT mechanism is retarded in CTAB medium. Upon encapsulation in other two micellar system the lactam emission intensity drastically enhances due to the conjugate effect of lower polarity and increase rigidity imposed by the micellar environments than the bulk aqueous phase. Both steady state and TCSPC results point towards the fact that the lactim–lactam conversion is favourable towards the lactam form in the anionic and neutral micellar medium. The results obtained from the micropolarity measurement at the probe interaction site within the micelles along with the results of fluorescence quenching experiments using Cu2+ and CPCl quencher suggest that the probable locations of ADII in the three micellar systems are: Stern layer for the cationic and anionic micellar systems but in anionic medium ADII is more exposed to the bulk aqueous phase (Gouy–Chapmann layer) whereas in nonionic micellar system the probe is probably localized in the palisade layer of the micellar system. The evaluation of binding constants of ADII with the studied micellar assemblies reveals the strongest binding affinity of ADII towards the neutral micellar system, TX-100 and in all three micellar assemblies the binding process is spontaneous. This result is found to corroborate well to the time resolved fluorescence anisotropic results suggesting the slowest rotational relaxational time of ADII in neutral micellar medium.In summary, studying the binding interaction of a biologically relevant molecule ADII with relevant biological and/or biomimicking receptors is very much essential because of its prospective biological and medicinal applications.
Acknowledgements
DR acknowledges University Grant Commission, India for senior research fellowship. NG likes to acknowledge UPE and CRNN of CU for financial support.References
- C. O. R. Yagui, A. Pessoa Jr and L. C. Tavares, J. Pharm. Pharm. Sci., 2005, 8, 147 Search PubMed.
- S. Gokturk, E. Calıskan, R. YesimTalman and U. Var, Sci. World J., 2012, 2012, 718791 Search PubMed.
- N. S. Bodor, Chemical Aspects of Drug Delivery Systems, ed. D. R. Karsa and R. A. Stephenson, Royal Society of Chemistry, London, 1996 Search PubMed.
- A. Techen, C. Hille, C. Dosche and M. U. Kumke, J. Colloid Interface Sci., 2012, 377, 251 CrossRef CAS PubMed.
- Y. Tamoto, H. Segawa and H. Shirota, Langmuir, 2005, 21, 3757 CrossRef CAS PubMed.
- N. C. Maiti, M. M. G. Krishna, P. J. Brito and N. Periasamy, J. Phys. Chem. B, 1997, 101, 11051 CrossRef CAS.
- B. Cohen, D. Huppert, K. M. Solntsev, Y. Tsfadia, E. Nachliel and M. Gutman, J. Am. Chem. Soc., 2002, 124, 7539 CrossRef CAS PubMed.
- D. Mandal, S. K. Pal and K. Bhattacharyya, J. Phys. Chem. A, 1998, 102, 9710 CrossRef CAS.
- K. J. Tielrooij, M. J. Cox and H. J. Bakker, ChemPhysChem, 2009, 10, 245 CrossRef CAS PubMed.
- E.-A. Gould, A. V. Popov, L. M. Tolbert, I. Presiado, Y. Erez, D. Huppert and K. M. Solntsev, Phys. Chem. Chem. Phys., 2012, 14, 8964 RSC.
- A. S. Klymchenko and A. P. Demchenko, Phys. Chem. Chem. Phys., 2003, 5, 461 RSC.
- R. Das, G. Duportail, L. Richert, A. Klymchenko and Y. Mély, Langmuir, 2012, 28, 7147 CrossRef CAS PubMed.
- R. Adhikary, P. J. Carlson, T. W. Kee and J. W. Petrich, J. Phys. Chem. B, 2010, 114, 2997 CrossRef CAS PubMed.
- N. Sarkar, A. Datta, S. Das and K. Bhattacharyya, J. Phys. Chem., 1996, 100, 15423 Search PubMed.
- D. Ray, A. Pramanik and N. Guchhait, J. Photochem. Photobiol., A, 2014, 274, 33 CrossRef CAS PubMed.
- A. F. Pozharskii, A. T. Soldatenkov and A. R. Katritzky, Heterocycles in Life and Society; John Wiley and Sons Ltd., New York, 1997 Search PubMed.
- E. A. Barnard and W. D. Stein, Adv. Enzymol. Relat. Subj. Biochem., 1958, 20, 51 CAS.
- M. Boiani and M. Gonzalez, Mini-Rev. Med. Chem., 2005, 5, 409 CrossRef CAS.
- Z. Jin, Nat. Prod. Rep., 2005, 22, 196 RSC.
- S. Das, R. Fro1hlich and A. Pramanik, Org. Lett., 2006, 8, 4263 CrossRef CAS PubMed.
- J. R. Lakowicz, Priniples of Fluorescence Spectroscopy, Plenum, New York, 3rd edn, 2006 Search PubMed.
- P. K. Das and A. Chaudhuri, Langmuir, 1999, 15, 8771 CrossRef CAS.
- E. Gaidamauskas, D. P. Cleaver, P. B. Chatterjee and D. C. Crans, Langmuir, 2010, 26, 13153 CrossRef PubMed.
- C. Esposito, P. Colicchio, A. Facchiano and R. Ragone, J. Colloid Interface Sci., 1998, 200, 310 CrossRef CAS.
- F. Geng, L. Yu, T. Lu, Z. Li, L. Zheng and G. Li, J. Dispersion Sci. Technol., 2008, 29, 1209 CrossRef CAS.
- O. Lopez, M. Cocera, R. Pons, N. Azemar, C. Lopez-Iglesias, E. Wehrli, J. L. Parra and A. de la Maza, Langmuir, 1999, 15, 4678 CrossRef CAS.
- B. K. Paul, D. Ray and N. Guchhait, J. Phys. Chem. B, 2012, 116, 9704 CrossRef CAS PubMed.
- S. Mandal, V. G. Rao, C. Ghatak, R. Pramanik, S. Sarkar and N. Sarkar, J. Phys. Chem. B, 2011, 115, 12108 CrossRef CAS PubMed.
- M. Almgren, F. Grieser and J. K. Thomas, J. Am. Chem. Soc., 1979, 101, 279 CrossRef CAS.
- K. Bhattachryya and M. Chowdhury, Chem. Rev., 2003, 93, 507 CrossRef.
- M. E. Vazquez, J. B. Blanco and B. Imperiali, J. Am. Chem. Soc., 2005, 127, 1300 CrossRef CAS PubMed.
- Y. Moroi, Micelles, Plennum, New York, 1992 Search PubMed.
- M. Kumbhakar, S. Nath, T. Mukherjee and H. Pal, J. Chem. Phys., 2004, 121, 6026 CrossRef CAS PubMed.
- G. Saroja and A. Samanta, Chem. Phys. Lett., 1995, 246, 506 CrossRef CAS.
- S. M. Dennison, J. Guharay and P. K. Sengupta, Spectrochim. Acta, Part A, 1999, 55, 903 CrossRef.
- K. Kalyansundram and J. K. Thomas, J. Phys. Chem., 1977, 81, 2176 CrossRef.
- B. K. Paul, A. Samanta and N. Guchhait, Langmuir, 2010, 26, 3214 CrossRef CAS PubMed.
- P. Banerjee, S. Pramanik, A. Sarkar and S. C. Bhattacharya, J. Phys. Chem. B, 2008, 112, 7211 CrossRef CAS PubMed.
- A. Mahata, D. Sarkar, D. Bose, D. Ghosh, A. Girigoswami, P. Das and N. Chattopadhyay, J. Phys. Chem. B, 2009, 113, 7517 CrossRef CAS PubMed.
- A. Samanta, B. K. Paul and N. Guchhait, J. Fluoresc., 2012, 22, 289 CrossRef CAS PubMed.
- S. C. Bhattacharya, H. T. Das and S. P. Moulik, J. Photochem. Photobiol., A, 1993, 71, 257 CrossRef CAS.
- A. P. Demchenko, Luminescence, 2002, 17, 19 CrossRef CAS PubMed.
- A. Samanta, J. Phys. Chem. B, 2006, 110, 13704 CrossRef CAS PubMed and references therein.
- A. Chattopadhyay and S. Mukherjee, Biochemistry, 1993, 32, 3804 CrossRef CAS.
- P. K. Mandal, A. Paul and A. Samanta, J. Photochem. Photobiol., A, 2006, 182, 113–120 CrossRef CAS PubMed.
- C. Ghatak, V. G. Rao, R. Pramanik, S. Sarkar and N. Sarkar, Phys. Chem. Chem. Phys., 2011, 13, 371120 RSC.
- B. K. Paul and N. Guchhait, Phys. Chem. Chem. Phys., 2012, 14, 8892–8902 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47322h |
| This journal is © The Royal Society of Chemistry 2014 |