Influence of CO2 on the stability of discharge performance for Li–air batteries with a hybrid electrolyte based on graphene nanosheets†
Eunjoo Yoo and
Haoshen Zhou*
Energy Technology Research Institute, National Institute of Advanced Industrial Science and Technology, Umezono 1-1-1, Central 2, Tsukuba, Ibaraki 305-8568, Japan. E-mail: hs.zhou@aist.go.jp; Fax: +81-29-861-5799; Tel: +81-29-861-5648
First published on 10th January 2014
Abstract
The long-term discharge performance of graphene nanosheets for Li–air batteries with hybrid electrolytes shows different behaviour depending on the surface state of graphene. The functional groups on graphene play a more important role than the defect sites for the formation of Li2CO3 and electrochemical performance in Li–air batteries with hybrid electrolytes.
Rechargeable Li–air batteries are expected to be the next generation of energy storage devices, because they have high energy densities and energy storage capacities compared with Li-ion batteries.1–3 Generally, in non-aqueous electrolyte Li–air batteries, during the discharge process, electrochemically reduced O2 on the surface of air cathodes combines with Li+ ions to form Li2O2 in a fundamentally different mechanism than in aqueous and hybrid electrolyte Li–air batteries, in which the Li+ ions react with OH to form LiOH.4,5 We have reported a Li–air battery with a hybrid electrolyte that overcomes the formation of Li2O2 and LiO2, which are insoluble in the electrolyte.6,7 However, there are some issues in the Li–air batteries with hybrid electrolytes especially when an alkaline solution is used as an electrolyte side cathode electrode. One major issue is the formation of Li2CO3 during the discharge process, because Li–air batteries with aqueous electrolytes have an open system which leads to the reaction of Li+ ions with CO2 in the air, resulting in the formation of Li2CO3. Precipitation of reaction products (such as Li2CO3) eventually blocks the path for oxygen into the air electrode and limits the capacity of Li–air batteries with aqueous electrodes. Therefore, there is a critical need to investigate the influence of CO2 in air on cell performance, for improving the cell lifetime of Li–air batteries with aqueous electrolytes.
Carbon materials such as carbon black, carbon nanotubes, graphene nanosheets, and acetylene black are used as catalysts and substrates for metal nanoparticles for electrocatalysts in Li–air batteries.7–10 Furthermore, the surface of carbon can vary between hydrophobic and hydrophilic, depending on the presence of functional groups, and may be influenced by the reactivity of carbon materials. Hence, understanding the relationship between the reaction products (such as Li2O2, LiO2 and Li2CO3) and the surface polarity of carbon is critical towards the realization of Li–air batteries. However, the formation of Li2CO3 in aqueous alkali electrolytes for Li–air batteries has still not been reported. Several recent reports have focused on the decomposition of carbon cathodes and electrolytes in non-aqueous Li–O2 batteries, resulting in the formation of Li2CO3.11,12 If the mechanism of Li2CO3 formation in Li–air batteries with aqueous alkali electrolyte systems is figured out, it can provide insight into ways to improve the electrochemical performance and durability of cell lifetime. We have also reported that the electrochemical performance of Li–air batteries using graphene nanosheets can be improved due to its specific morphology and defect sites.5 Although the graphene nanosheets are expected to be promising electrocatalysts for Li–air batteries, there are various kinds of graphene nanosheets with different surface areas, morphologies and surface states. Thus, it is important to clarify what kind of graphene nanosheet is more influenced by CO2 for Li–air batteries with hybrid electrolytes.
In this study, we report the influence of CO2 on the cell lifetime in Li–air batteries with hybrid electrolytes based on graphene nanosheets with functional groups. Commercial graphene sheets with different surface areas and surface states are also used as a reference under the same conditions to compare the electrode performance.
The specific surface areas of the graphene nanosheets used in this study are measured by using a multi-point Brunauer–Emmett–Teller (BET) method. The specific surface area is estimated to be 820.5 and 204.3 m2 g−1 for commercial graphene sheets and graphene nanosheets, respectively [see ESI, Fig. S1†]. The morphological structure of the graphene nanosheets and commercial graphene sheets was examined by SEM. The graphene nanosheets and commercial graphene sheets have a curled morphology with a wrinkled, paper-like structure [see ESI, Fig. S2†].
Fig. 1 shows the structure of a Li–air battery with a hybrid electrolyte. The graphene nanosheets contain many defect sites and oxygen containing functional groups used as catalysts in this study. These defect sites and functional groups can effectively serve as active sites for oxygen reduction reactions as well as nucleation sites for the formation of Li2CO3.
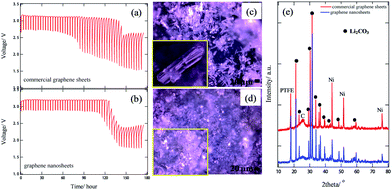
Fig. 2(a) and (b) show the long-term discharge performance of graphene nanosheets and commercial graphene sheets as a function of the CO2 concentration in the feed gas. To identify the effect of CO2 on Li–air batteries with hybrid electrolytes, the cell is discharged under two different atmospheres: air (about 400 ppmCO2/O2), and 1000 ppmCO2/O2. Using air as the feed gas, the cathode electrode is able to run for about 80 and 140 h for commercial graphene sheets and graphene nanosheets, respectively. Therefore, the lifetime of the cathode electrode is stable for 20 and 40 h for commercial graphene sheets and graphene nanosheets, respectively in oxygen containing 1000 ppmCO2. Interestingly, the graphene nanosheets exhibit good long-term discharge performance compared with the commercial graphene sheets. It is also clearly shown that the discharge lifetime of Li–air batteries with hybrid electrolytes strongly depends on the carbon materials. To investigate the reason for cathode potential loss after long-term discharge performance, optical microscopy and XRD measurements were carried out. The optical images show the deposited Li2CO3 covering the carbon surface of both samples. The XRD result also clearly exhibits the formation of Li2CO3 [see ESI, Fig. S3†]. We also examined the XPS measurement of commercial graphene sheets and graphene nanosheets before and after the discharge process, to examine the features of lithium species in carbon materials [see ESI, Fig. S4†]. The spectra showed that the O1s and Li1s peaks from Li2CO3 are located at 531.8 and 45.8 eV, respectively, after the discharge process, which is in agreement with the reported value for Li2CO3 (532.2 and 55 eV).13 Thus, it is considered that this cathode potential loss for both samples after the discharge process is attributed to the Li2CO3 precipitation, since insoluble Li2CO3 solid constituted physical barriers for mass transfer.
We also investigated the specific discharge capacity based on the weight of the cathode electrode (graphene nanosheets (90%) + AB (3%) + PTFE (7%)), in which AB was used as a conductive agent and PTFE used as a binder. Fig 2(c) shows the specific discharge capacity of graphene nanosheets and commercial graphene sheets. The specific discharge capacity of graphene nanosheets is achieved at 4800 mA h g−1 in air, while the commercial graphene sheets show a specific discharge capacity of about 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mA h g−1 in air. Furthermore, in oxygen containing 1000 ppmCO2, the discharge capacity of commercial graphene sheets is about 2 times higher than that of the graphene nanosheets. It is considered that the large discharge capacity of commercial graphene sheets is attributed to high specific surface areas as well as good electronic conductivity, which removed functional groups from the graphene sheets as discussed later in this work.
000 mA h g−1 in air. Furthermore, in oxygen containing 1000 ppmCO2, the discharge capacity of commercial graphene sheets is about 2 times higher than that of the graphene nanosheets. It is considered that the large discharge capacity of commercial graphene sheets is attributed to high specific surface areas as well as good electronic conductivity, which removed functional groups from the graphene sheets as discussed later in this work.
We also carried out a galvanostatic intermittent titration technique (GITT), which is a quasi-equilibrium technique, to study the discharge kinetics of Li–air batteries. Fig. 3(a) and (b) show the discharge potential as a function of discharge time obtained by GITT with a current density of 0.5 mA cm−2, followed by an open circuit voltage (OCV) relaxation for 3 h for both samples in air. There is an equilibrium plateau at 3.2 V for both samples, suggesting that the major plateaus at 3.2 V are due to the reaction of O2 + 4Li + 6H2O ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) 4(LiOH·H2O) (3.45 V).6,7 Additionally, the discharge potentials are stable at 2.73 and 2.82 V for about 80 and 120 h for commercial graphene sheets and graphene nanosheets, respectively. These results are in agreement with the long-term discharge performance (Fig. 2).
4(LiOH·H2O) (3.45 V).6,7 Additionally, the discharge potentials are stable at 2.73 and 2.82 V for about 80 and 120 h for commercial graphene sheets and graphene nanosheets, respectively. These results are in agreement with the long-term discharge performance (Fig. 2).
Fig. 3(c) and (d) show the optical images of both samples after GITT to observe the Li2CO3 deposited on the cathode electrode. Crystalline Li2CO3 has formed over the whole air electrode surface. Interestingly, we found that the morphology of the Li2CO3 crystals is different depending on the carbon materials. The commercial graphene sheets contain nanorod-like Li2CO3, whereas an irregular-shaped Li2CO3 is presented on the graphene nanosheets as shown in Fig. 3(c) and (d). The significant difference in the growth of Li2CO3 for graphene nanosheets and commercial graphene sheets suggests that the surface state of both samples is different, because the growth of the crystal is related to limiting the active nucleation sites such as defects and functional groups on the surface.14 Furthermore, the reaction product, Li2CO3, is also confirmed by XRD measurement as shown in Fig. 3(e). The XRD results of both samples after GITT show the peaks corresponding to Li2CO3.
It is well known that the defective sites, edge and functional groups of carbon materials are used as active sites as well as nucleation sites due to their high surface energy.14,15 Thus, understanding the roles of the defect sites and functional groups presented on carbon surfaces is very important for Li–air batteries to improve their electrochemical performance.
Fig. 4(a) shows typical TG curves of graphene nanosheets and commercial graphene sheets that are used to measure the amount of functional groups on the carbon surface. TG data of obtained graphene nanosheets exhibit a mass loss of 45% in the temperature range of RT to 500 °C, which resulted from the removal of residual oxygen containing functional groups on the graphene nanosheets. However, the commercial graphene sheets started combusting at about 400 °C. Moreover, from the elemental analysis results, as shown in Fig. 4(b), the graphene nanosheets have a C/O ratio of 1.7, while the C/O ratio of the commercial graphene sheets increases to 6.42. The C/O ratio reflects the relative content of defects and functional groups on graphene nanosheets, because the degree of defect formation and surface functionalization on carbon materials is correlative with the C/O ratio.16,17 Thus, these results prove the higher number of oxygen containing functional groups that are presented on the graphene nanosheets. J. Xiao et al. have suggested that both defect sites and functional groups increase the interfacial bonding and prevent agglomeration by DFT calculation.15 They also reported that the graphene with COOH groups has a stronger binding energy (−0.9 eV) than that of graphene without COOH groups (−0.70 eV). So it is favourable to nucleate around the defective sites with functional groups in graphene nanosheets and form the isolated particles.15 Fig. 4(c) shows the SEM images of graphene nanosheets and commercial graphene sheets after discharging for 150 and 100 h, respectively, in air. On the graphene nanosheets with a C/O ratio of 1.7, the particle sizes of Li2CO3 are 20–30 nm and the Li2CO3 particles are distributed homogeneously on graphene nanosheets, indicating that the aggregation of Li2CO3 is prevented. For commercial graphene sheets with a C/O ratio of 6.4, larger Li2CO3 particles are observed (20–120 nm) and the particle size distribution is less homogeneous. These observations indicate that the difference in nucleation and growth of Li2CO3 is likely related to the amount of functional groups on the graphene nanosheets.
We propose a possible reason to account for the difference in long-term discharge performances based on the surface state of graphene. As discussed above, the deposited Li2CO3 on graphene nanosheets has relatively strong interactions between Li2CO3 cluster and defect sites with functional groups of graphene. Furthermore, the Li2CO3 particles are distributed homogeneously on graphene nanosheets, leading to effectively diffused oxygen gas toward the cathode electrode during the discharge process. Unlike the graphene nanosheets, the Li2CO3 formed on the commercial graphene sheets favours aggregation of the Li2CO3 particles due to the limited number of active nucleation sites, resulting in physical barriers for mass transfer. However, it is not yet clearly understood. We therefore consider that the functional groups presented on carbon surfaces play more important roles than the defect sites for the formation of Li2CO3 and electrochemical performance in Li–air batteries with hybrid electrolyte systems. If it is possible to control the defect sites and functional groups on carbon surfaces, such surface control could be highly advantageous for metal–air batteries.
Conclusions
We demonstrate the long-term discharge performance of Li–air batteries with hybrid electrolytes based on graphene nanosheets, with different surface states used as cathode electrodes. The graphene nanosheets with a number of oxygen containing functional groups exhibit good discharge durability in comparison with commercial graphene sheets with few functional groups. This result indicates that the control of the surface chemistry of carbon electrodes is very important for improving the performance of Li–air batteries. In particular, the critical factor is related to the Li2CO3 deposition and growth mechanism of Li2CO3 on graphene nanosheets. Because the Li2CO3 formed around defects with functional groups on graphene nanosheets, oxygen transfer is enhanced during the discharge process, resulting in improved cell lifetime. We expect that this study can efficiently provide information for the relationship between electrochemical performance and surface chemistry for carbon electrodes in Li–air battery systems.Acknowledgements
This work was partially supported by the Grant for Science and Technology Research from Suzuki Foundation. LISICON (lithium super-ion conductor glass film) was provided by the Ohara Company in Japan.Notes and references
- A. Armand and J. M. Tarascon, Nature, 2008, 451, 652 CrossRef PubMed.
- K. M. Abraham and Z. Jiang, J. Electrochem. Soc., 1996, 143, 1 CrossRef CAS PubMed.
- T. Ogasawara, A. Dé bart, M. Holzapfel, K. P. Nová and P. G. Bruce, J. Am. Chem. Soc., 2006, 128, 1390 CrossRef CAS PubMed.
- H. He, W. Niu, N. M. Asl, J. Salim, R. Chen and Y. Kim, Electrochim. Acta, 2012, 67, 87 CrossRef CAS PubMed.
- E. J. Yoo and H. Zhou, J. Power Sources, 2013, 244, 429 CrossRef CAS PubMed.
- Y. Wang and H. Zhou, J. Power Sources, 2010, 195, 358 CrossRef CAS PubMed.
- E. J. Yoo and H. Zhou, ACS Nano, 2011, 5, 3020 CrossRef CAS PubMed.
- S. Nakanishi, F. Mizuno, K. Nobuhara, T. Abe and H. Iba, Carbon, 2012, 50, 4794 CrossRef CAS PubMed.
- D. Zhai, H. H. Wang, J. Yang, K. C. Lau, K. Li, K. Amine and L. A. Curtiss, J. Am. Chem. Soc., 2013, 135, 15364 CrossRef CAS PubMed.
- Y. Li, J. Wang, X. Li, D. Geng, M. N. Banis, R. Li and X. Sun, Electrochem. Commun., 2012, 18, 12 CrossRef CAS PubMed.
- H. K. Lim, H. D. Lim, K. Y. Park, D. H. Seo, H. G. Gwon, J. Hong, W. A. Goddard, H. Kim and K. Kang, J. Am. Chem. Soc., 2013, 135, 9733 CrossRef CAS PubMed.
- B. D. McCloskey, D. S. Bethune, R. M. Shelly, G. Girishkumar and A. C. Luntz, J. Phys. Chem. Lett., 2011, 2, 1161 CrossRef CAS.
- Y. C. Lu, A. N. Mansour, N. Yabuuchi and Y. Shao-Horn, Chem. Mater., 2009, 21, 4408 CrossRef CAS.
- R. Wen, M. Hong and H. R. Byon, J. Am. Chem. Soc., 2013, 135, 10870 CrossRef CAS PubMed.
- J. Xiao, D. Mei, X. Li, W. Xu, D. W. G. L. Graff, W. D. Bennett, Z. Nie, L. V. Saraf, I. A. Aksay, J. Liu and J. G. Zhang, Nano Lett., 2011, 11, 5071 CrossRef CAS PubMed.
- K. N. Kudin, B. Ozbas, H. C. Schniepp, R. K. Prudhomune, I. A. Aksay and R. Car, Nano Lett., 2008, 8, 36 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47258b |
| This journal is © The Royal Society of Chemistry 2014 |