Cyclic arginyl–glycyl–aspartic acid (RGD) peptide-induced synthesis of uniform and stable one-dimensional CdTe nanostructures in aqueous solution†
Hua Hea,
Xing Suna,
Xiaojuan Wanga,
Yawei Suna,
Hai Xu*a and
Jian R. Lu*b
aCentre for Bioengineering and Biotechnology, China University of Petroleum (East China), 66 Changjiang West Road, Qingdao Economic Development Zone, Qingdao, 266580, P. R. China. E-mail: xuh@upc.edu.cn; Fax: +86-532-86981569; Tel: +86-532-86981569
bBiological Physics Laboratory, School of Physics and Astronomy, University of Manchester, Schuster Building, Manchester M13 9PL, UK. E-mail: j.lu@manchester.ac.uk; Fax: +44 (0)161-2003926; Tel: +44 (0)161-2003926
First published on 14th February 2014
Abstract
Aqueous synthesis of one dimensional (1D) semiconductor nanostructures presents a great challenge. By using a cyclic RGD peptide as the ligand, 1D wurtzite CdTe nanocrystals with uniform size and excellent storage stability were prepared in aqueous solution. The integration of one dimensionality with the specific RGD motif makes the CdTe nanocrystals attractive in constructing optical and electrical devices or sensors for biotechnological applications.
One-dimensional (1D) II–VI semiconductor nanocrystals (e.g. nanorods and nanowires) are of interest because of their unique optical and electrical properties and many potential technical applications.1 The preparation of uniform and stable 1D CdTe (or CdSe) nanostructures through wet chemistry has been actively explored in the past two decades.2,3 Although high quality 1D CdTe nanocrystals could be prepared by lipophilic organometallic precursors in hot organic solvents in the presence of two or more ligands,4 these organic synthesis routes typically involved expensive and hazardous agents and harsh experimental conditions. As an alternative, aqueous synthesis might alleviate or avoid these disadvantages.5 But the direct aqueous synthesis of 1D semiconductor nanocrystals has been less developed due to multifold reasons such as low reaction temperature and water interference.6–10 Tang et al. reported the construction of 1D CdTe nanostructures in aqueous solution by self-organization of CdTe nanoparticles after partially removing their capping ligands.7 Li et al. first observed the aqueous growth of 1D CdTe nanorods in a mixed ligand system of cysteine and thioglycolic acid (TGA).8 Zhang et al. then demonstrated that only TGA and its derivatives could lead to the formation of 1D CdTe nanocrystals in aqueous solution.9 Moreover, they found that TGA or its derivative-capped 1D CdTe nanocrystals degraded gradually into nanoparticles over two weeks of storage.9 In this case, the addition of a secondary ligand, 1-thioglycerol (TG) or 2-mercaptoethylamine (MA), was required to afford structural stability. Overall, it still remains an important subject of synthetic chemistry to prepare stable and uniform 1D CdTe nanostructures in aqueous solution.
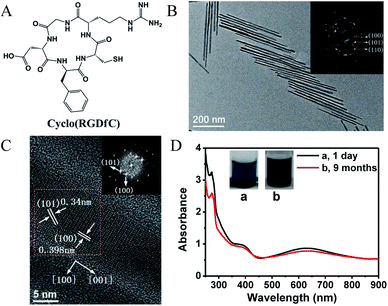
In this communication, we report the growth and control of 1D CdTe nanostructures with uniform size and excellent storage stability by using a RGD peptide as the ligand. The growth of CdTe nanocrystals was based on the reaction of cadmium chloride (CdCl2) and sodium hydrotelluride (NaHTe) at 98 °C, with detailed experimental procedures in ESI.† The used RGD peptide is a cyclic pentapeptide, Arg–Gly–Asp–D-Phe–Cys, abbreviated as cyclo(RGDfC) (Fig. 1A). Note that cyclo(RGDfC) is a commercial bioactive peptide carrying the RGD motif, which can specifically recognize a cell surface receptor integrin αvβ3.11 RGD-capped semiconductor nanocrystals are thus attractive in constructing optical and electrical devices or sensors with a well defined biointerfacial function.12 A representative transmission electron microscopy (TEM) image of the reaction solution (0.4 mM Cd2+, Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Te
Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclo(RGDfC) = 2
cyclo(RGDfC) = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1 h of heating) is shown in Fig. 1B, indicating the formation of uniform 1D CdTe nanostructures, whose lengths and diameters are 413 nm and 12.8 nm, respectively. The selected area electron diffraction (SAED) analysis (Fig. 1B inset) confirmed the crystalline nature.
5, 1 h of heating) is shown in Fig. 1B, indicating the formation of uniform 1D CdTe nanostructures, whose lengths and diameters are 413 nm and 12.8 nm, respectively. The selected area electron diffraction (SAED) analysis (Fig. 1B inset) confirmed the crystalline nature.
The interplanar spacings calculated from the diffraction rings are 3.98, 3.40 and 2.29 Å, consistent with those of the lattice planes (100), (101), (110) of the hexagonal wurtzite CdTe structures. Well-resolved lattice planes in the high resolution TEM (HRTEM) image indicate again their good crystallinity (Fig. 1C). The lattice plane spacings calculated from the fast Fourier transform (FFT) pattern are also 3.98 Å hkl (100) and 3.40 Å hkl (101) (Fig. 1C inset). The perpendicular orientations ([100] and [110]) of the (100) and (110) planes with respect to the [001] direction, as well as the slanted (101) plane projection suggest that the anisotropic growth of the 1D CdTe nanostructures occurs along the [001] direction of wurtzite crystal lattice (Fig. 1C and S1†).7 Because of the elongation of the CdTe nanostructures, the resulting solution gave dark blue colour with characteristic optical absorption at around 605 nm, as shown in Fig. 1D. In contrast, solutions of spherical CdTe nanoparticles were light yellow or yellow, depending on particle sizes (see Fig. S2†). This feature allows us to make a rapid and qualitative judgment about the formation of 1D CdTe nanostructures. Note that their luminescence was hard to observe due to the poor quantum confinement of 1D nanostructures compared to 0D nanostructures.9,13
Furthermore, the cyclo(RGDfC)-capped 1D CdTe nanocrystals showed excellent structural stability over more than nine months of storage at 4 °C (or longer). There was little variation in solution colour and absorption spectra after nine-months, as shown in Fig. 1D. Their lengths and diameters remained nearly unchanged as determined by TEM (see Fig. S3†). It is evident that the bioactive cyclo(RGDfC) ligand has obvious advantage in producing stable 1D CdTe nanocrystals, in comparison with the existing TGA or TGA-like ligands.9
As the anisotropic growth of nanocrystals is related to the precursor or monomer concentration, the growth and morphology of the CdTe nanostructures were thus regulated by varying the precursor ratio and concentration. Specifically, we here controlled the Cd/Te molar ratio by adding different amounts of NaHTe into the solution with a fixed Cd2+-cyclo(RGDfC) concentration (cyclo(RGDfC)/Cd2+ = 2.5/1, Cd2+ = 0.4 mM). Fig. 2A shows absorption spectra and optical photographs of the resulting CdTe solutions at the precursor Cd/Te ratios of 4/0.5, 4/0.75, 4/1, 4/2 and 4/4 after 1 h of heating, with their TEM images shown in ESI (see Fig. S2 and S4†). At a high Cd/Te ratio of 4/0.5, dot-shaped nanoparticles rather than 1D nanostructures were produced irrespective of the heating time. In this case, the longer the heating time, the larger the dot-shaped particles grew with a solution colour from light yellow to yellow (Fig. S2†). With increasing the Te precursor concentration, the resultant CdTe solution deepened gradually in colour from nearly colourless to light blue and then dark blue, accompanied by the occurrence of the peak around 605 nm and the increase in its intensity. The colour deepening and the absorption increase of the CdTe solution was presumably attributed to the formation of more 1D CdTe nanocrystals. But more 1D CdTe nanostructures do not imply continuous growth in their lengths. For example, as the Cd/Te precursor ratio was reduced from 4/0.75 to 4/1, the length of the 1D CdTe nanostructures increased from some 322 nm to 428 nm; with further reduction to 4/2 and 4/4, however, the length decreased to some 413 nm and 218 nm, respectively (see Fig. S4†). This is reasonable because relatively higher HTe− concentrations might lead to more nuclei formed during the nucleation stage, thus resulting in considerable reduction of precursors or monomers during the crystal growth and finally being unfavourable for the anisotropic growth of nanocrystals.2 The result of varying the precursor ratio suggests an optimal ratio (i.e. Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Te
Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclo(RGDfC) = 2
cyclo(RGDfC) = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) in terms of the length and uniformity of 1D CdTe nanostructures. At this ratio, the crystal growth was found to be closely related to the precursor concentration. Fig. 2B shows absorption spectra and photographs of CdTe solutions with the Cd precursor concentration at 0.2 mM and 2 mM, respectively. Only dot-shaped nanoparticles were produced at the low precursor concentration (Fig. 2C). Upon increasing Cd2+ to 2 mM, the resultant 1D CdTe nanocrystals elongated with their length reaching more than 600 nm (Fig. 2D).
5) in terms of the length and uniformity of 1D CdTe nanostructures. At this ratio, the crystal growth was found to be closely related to the precursor concentration. Fig. 2B shows absorption spectra and photographs of CdTe solutions with the Cd precursor concentration at 0.2 mM and 2 mM, respectively. Only dot-shaped nanoparticles were produced at the low precursor concentration (Fig. 2C). Upon increasing Cd2+ to 2 mM, the resultant 1D CdTe nanocrystals elongated with their length reaching more than 600 nm (Fig. 2D).
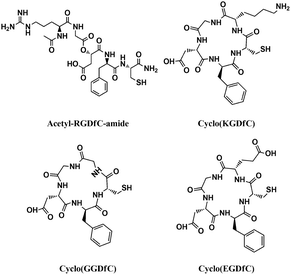
Surface ligands play important roles on the growth and stability of 1D CdTe nanostructures.4a,9 Previous reports have demonstrated that only TGA or TGA-like ligands (e.g. thiolactic acid (TLA) and L-cysteine (LCS)) allowed the anisotropic growth of wurtzite CdTe nanocrystals in aqueous solution at 80 °C and over certain precursor concentrations. The presence of 3-mercaptopropionic acid (MPA), TG and MA only produced dot-shaped zinc-blende nanocrystals. These results are indicative of the importance of both carbonyl and thiol groups as well as their relative position for the anisotropic growth of CdTe nanocrystals. However, the resultant 1D CdTe nanostructures in the presence of one or two TGA and TGA-like ligands degraded into dot-shaped nanoparticles within two weeks of storage.9 To improve their stability, different ligands such as TG or MA were required. In this study, the formation of 1D wurtzite CdTe nanostructures with excellent stability in the presence of only cyclo(RGDfC) must benefit from its specific peptide sequence and steric structure. To confirm this point, we examined the growth of CdTe in the presence of linear acetyl-RGDfC-amide, cyclo(KGDfC), cyclo(GGDfC), and cyclo(EGDfC). Fig. 3 presents the molecular structures of these peptides. Upon elimination of the cyclic structure, only a small amount of irregular 1D CdTe nanostructures was produced in the presence of linear acetyl-RGDfC-amide (Fig. 4A). With a cyclic structure, however, the variations in peptide sequence also disabled the anisotropic growth of the CdTe nanostructures. For example, when arginine (R) was replaced by glycine (G), and lysine (K) by glutamic acid (E), respectively, we only observed the formation of quasi-spherical CdTe nanoparticles (Fig. 4B–D). Interestingly, we observed here that only Cd-cyclo(RGDfC) solution has thick and turbid white precipitates at the pH range of 6 to 9 (before the addition of NaHTe), in sharp contrast to clear and transparent Cd solutions containing other peptides under the same conditions (see Fig. S5†). This might imply a unique Cd-cyclo(RGDfC) complex favouring the anisotropic growth. Furthermore, Fourier transform infrared (FTIR) spectra of cyclo(RGDfC), acetyl-RGDfC-amide, and the corresponding CdTe nanostructures confirmed that in addition to the thiol group, the guanidine and amide groups in the cyclic structure were also involved in the formation and stabilization of 1D CdTe nanostructures (see Fig. S6†).
In conclusion, we have developed the process for the uniform growth of the 1D CdTe nanostructures with robust structural stability in aqueous solution by using the RGD peptide with a cyclic structure as the ligand. The length of the 1D CdTe nanocrystals could be well regulated by varying the precursor ratio and concentration. More importantly, the integration of the 1D semiconductor nanostructures with a biologically functional moiety (RGD) is important in constructing optical and electrical devices or sensors for technical applications. Relevant studies are underway in our laboratory.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (20905078, 21103230), the Natural Science Foundation of Shandong Province (ZR2009BQ001, JQ201105), and the Fundamental Research Funds for the Central Universities (12CX04053A, 11CX05001A).Notes and references
- (a) W. U. Huynh, J. J. Dittmer and A. P. Alivisatos, Science, 2002, 295, 2425 CrossRef CAS PubMed; (b) X. Duan, Y. Huang, R. Agarwal and C. M. Lieber, Nature, 2003, 421, 241 CrossRef CAS PubMed; (c) M. Law, L. E. Greene, J. C. Johnson, R. Saykally and P. Yang, Nat. Mater., 2005, 4, 455 CrossRef CAS PubMed; (d) T. Mokari, E. Rothenberg, I. Popov, R. Costi and U. Banin, Science, 2004, 304, 1787 CrossRef CAS PubMed; (e) J. Jie, W. Zhang, I. Bello, C. S. Lee and S. T. Lee, Nano Today, 2010, 5, 313 CrossRef CAS PubMed.
- (a) X. Peng, L. Manna, W. Yang, J. Wickham, E. Scher, A. Kadavanich and A. P. Alivisatos, Nature, 2000, 404, 59 CrossRef CAS PubMed; (b) Z. A. Peng and X. Peng, J. Am. Chem. Soc., 2002, 124, 3343 CrossRef CAS PubMed; (c) Z. A. Peng and X. Peng, J. Am. Chem. Soc., 2001, 123, 1389 CrossRef CAS; (d) L. Manna, E. C. Scher and A. P. Alivisatos, J. Am. Chem. Soc., 2000, 122, 12700 CrossRef CAS.
- (a) S. Kumar and T. Nann, Small, 2006, 2, 316 CrossRef CAS PubMed; (b) S. Srivastava, A. Santos, K. Critchley, K. S. Kim, P. Podsiadlo, K. Sun, J. Lee, C. L. Xu, G. D. Lilly, S. C. Glotzer and N. A. Kotov, Science, 2010, 327, 1355 CrossRef CAS PubMed; (c) H. J. Niu, L. W. Zhang, M. Y. Gao and Y. M. Chen, Langmuir, 2005, 21, 4205 CrossRef CAS PubMed; (d) S. Kumar, M. Ade and T. Nann, Chem.–Eur. J., 2005, 11, 2220 CrossRef CAS PubMed.
- (a) W. W. Yu, Y. A. Wang and X. Peng, Chem. Mater., 2003, 15, 4300 CrossRef CAS; (b) L. Manna, D. J. Milliron, A. Meisel, E. C. Scher and A. P. Alivisatos, Nat. Mater., 2003, 2, 382 CrossRef CAS PubMed; (c) M. Kuno, O. Ahmad, V. Protasenko, D. Bacinello and T. H. Kosel, Chem. Mater., 2006, 18, 5722 CrossRef CAS; (d) Y. H. Liu, F. Wang, J. Hoy, V. L. Wayman, L. K. Steinberg, R. A. Loomis and W. E. Buhro, J. Am. Chem. Soc., 2012, 134, 18797 CrossRef CAS PubMed; (e) F. Jiang, J. Liu, Y. Li, L. Fan, Y. Ding and Y. Li, Adv. Funct. Mater., 2012, 22, 2402 CrossRef CAS.
- (a) N. Gaponik, D. V. Talapin, A. L. Rogach, K. Hoppe, E. V. Shevchenko, A. Kornowski, A. Eychmuller and H. Weller, J. Phys. Chem. B, 2002, 106, 7177 CrossRef CAS; (b) A. L. Rogach, T. Franzl, T. A. Klar, J. Feldmann, N. Gaponik, V. Lesnyak, A. Shavel, A. Eychmueller, Y. P. Rakovich and J. F. Donegan, J. Phys. Chem. C, 2007, 111, 14628 CrossRef CAS; (c) L. Li, H. Qian and J. Ren, Chem. Commun., 2005, 528 RSC; (d) H. He, H. Qian, C. Dong, K. Wang and J. Ren, Angew. Chem., Int. Ed., 2006, 45, 7588 CrossRef CAS PubMed.
- Y. L. Li, L. H. Jing, R. R. Qiao and M. Y. Gao, Chem. Commun., 2011, 47, 9293 RSC.
- Z. Y. Tang, N. A. Kotov and M. Giersig, Science, 2002, 297, 237 CrossRef CAS PubMed.
- J. Li, X. Hong, D. Li, K. Zhao, L. Wang, H. Wang, Z. Du, J. Li, Y. Bai and T. Li, Chem. Commun., 2004, 1740 RSC.
- (a) H. Zhang, D. Y. Wang and H. Möhwald, Angew. Chem., Int. Ed., 2006, 45, 748 CrossRef CAS PubMed; (b) H. Zhang, D. Wang, B. Yang and H. Möhwald, J. Am. Chem. Soc., 2006, 128, 10171 CrossRef CAS PubMed.
- (a) D. W. Deng, Y. B. Qin, X. Yang, J. S. Yu and Y. Pan, J. Cryst. Growth, 2006, 296, 141 CrossRef CAS PubMed; (b) V. Sgobba, C. Schulz Drost and D. M. Guldi, Chem. Commun., 2007, 565 RSC; (c) P. Yang, M. Ando and N. Murase, Adv. Mater., 2009, 21, 4016 CrossRef CAS; (d) T. Wang, Z. Jin, Y. Shi, W. Li and J. Yang, Cryst. Growth Des., 2009, 9, 5077 CrossRef CAS.
- J. P. Xiong, T. Stehle, R. Zhang, A. Joachimiak, M. Frech, S. L. Goodman and M. A. Arnaout, Science, 2002, 296, 151 CrossRef CAS PubMed.
- (a) W. Cai and X. Chen, Nat. Protoc., 2008, 3, 89 CrossRef CAS PubMed; (b) H. He, M. Feng, J. Hu, C. Chen, J. Wang, X. Wang, H. Xu and J. R. Lu, ACS Appl. Mater. Interfaces, 2012, 4, 6362 CrossRef CAS PubMed.
- H. Yu, J. Li, R. A. Loomis, L. W. Wang and W. E. Buhro, Nat. Mater., 2003, 2, 517 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and Fig. S1–S6. See DOI: 10.1039/c3ra47253a |
| This journal is © The Royal Society of Chemistry 2014 |