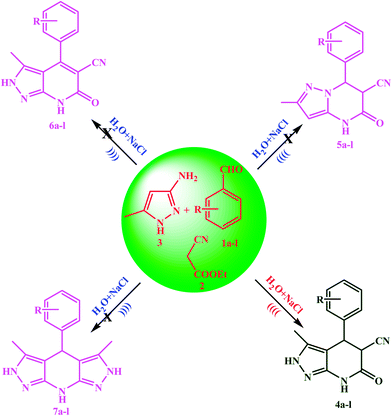
An efficient ultrasound-assisted one-pot chemoselective synthesis of pyrazolo[3,4-b] pyridine-5-carbonitriles in aqueous medium using NaCl as a catalyst†
Anshu Dandia*,
Shyam L. Gupta and
Vijay Parewa
Centre of Advanced Studies, Department of Chemistry, University of Rajasthan, Jaipur-302004, India. E-mail: dranshudandia@yahoo.co.in
First published on 6th January 2014
Abstract
A simple, convenient and green synthetic protocol for the chemoselective synthesis of pyrazolo[3,4-b]pyridine derivatives via three-component reaction of 3-amino-5-methylpyrazole, ethyl cyanoacetate, and aldehydes catalyzed by sodium chloride under ultrasound irradiation in aqueous medium is described. The method showed remarkable selectivity for pyrazolo[3,4-b]pyridine over dehydrogenated pyrazolopyridines, bis-pyrazolopyridines and pyrazolo[1,5-a]pyrimidine derivatives. The reactions were carried out under both conventional and ultrasonic irradiation conditions. Under ultrasound radiation, the catalytic activity of NaCl was about 15-fold higher as compared to the conventional method. The sonochemical procedure offers several advantages including cleaner reaction profile, use of easily available, cheap and environmentally benign catalyst, high yields, and simple experimental and work-up procedures.
1. Introduction
A primary driver of synthetic organic chemistry is the development of efficient and environmentally benign synthetic protocols. As the pressure to produce the myriad of substances required by society in an environmentally benign fashion has continued to increase, ultrasound-assisted chemistry has emerged as a discipline that permeates all aspects of synthetic chemistry.1 The major goals of this endeavor are to maximize the efficient use of safer raw materials and to reduce waste. Ultrasound has been used in the synthesis of many organic chemicals for years.2 The presence of ultrasound has shown to enhance the rates of reactions by manifolds as compared to its absence. In the presence of ultrasound, reactions have also been reported to occur at less severe conditions and in the presence of less expensive and less active materials as catalysts as compared to their silent counterparts (approach without the use of ultrasound).3,4 Control of selectivity is among the most important objectives in organic chemistry. For multicomponent reactions5 involving the simultaneous molecular interaction of three or more components, the issue of selectivity is of significance due to the high probability of several parallel reaction pathways leading to different products6,7 but many parameters such as temperature, pressure, solvent, catalyst type, kinetic or thermodynamic control, and other factors can be utilized to modulate the selectivity of synthetic transformations.Pyrazolopyridines are the promising class of heterocyclic compounds which inhibits cyclin-dependent protein kinase-2 (cdk-2), cyclin-dependent protein kinase-5 (cdk-5), and phosphatidylinositol 3-kinase (PI3-K).8–10 Thus, these compounds have potential for the treatment of several diseases including bipolar disorder, diabetes, dementia, Alzheimer's disease, schizophrenia, depression, and cancer.11 Some examples of pyrazolopyridine derivatives are anxiolytic drugs such as cartazolate,11 etazolate12 and tracazolate13 etc. Besides pyrazolopyridine derivatives also have industrial importance as fluorescence and luminophores in organic light emitting diodes.14 These examples emphasize the importance of pyrazolopyridines, as key pharmacophores in bioactive small molecules. The survey of literature revealed that pyrazolopyridines have been synthesized by employing number of methods such as by the reaction of phenylhydrazine, 3-aminocrotononitrile, isatin/acenaphthylene-1,2-dione, cyclic 1,3-dicarbonyl compounds viz. cyclohexane-1,3-diones, barbituric acid and 2-thioxodihydropyrimidine-4,6(1H,5H)-dione in aqueous media and catalyzed by (±)-camphor-10-sulfonic acid (CSA),15a by three-component reaction of 5-aminopyrazoles, isatin and cyclic β-diketones in aqueous media catalyzed by p-TSA,15b by the reaction of 5-(4-R-benzylamino)pyrazoles, cyclic β-diketones and paraformaldehyde under microwave irradiation,15c by the reaction of aldehyde, 3-amino-5-methylpyrazole and ethyl cyanoacetate in ethanol using a catalytic amount of p-toluenesulfonic acid,15d by the reaction of 6-aminopyrimidine-4-ones with dimedone and formaldehyde solution using triethylamine as a basic catalyst,15e by the reaction of aldehyde, acyl acetonitrile, and amino heterocycles in ionic liquid,15f but all these methods suffered from drawbacks such as longer reaction time, lower yield, use of hazardous organic solvents and reagents, tedious workup procedures, and co-occurrence of several side reactions with less selectivity of the process. Thus, the search for new catalyst and methods is still of growing importance.
Most of the catalyst are somewhat exotic, expensive, harmful and even uneffective in the absence of acidic additives. Herein we report the “yet-another-one-catalyst” idea to absurdity by proposing NaCl promotes the reaction that actually requires no catalyst, neither rare nor expensive.16 However, the use of some other metal catalysts are expensive, toxic, unavailable, metal containing catalyst which is harmful for human as well as environment and separation process also tedious, comparatively NaCl very cheap, easily available, harmless and most accessible (hence superior) catalyst for the chemoselective synthesis of pyrazolopyridine derivatives.
As a consequence of our interest in ultrasonic-assisted organic synthesis (UAOS)17 and our continued work on the synthesis of heterocyclic derivatives18 guided by the observation that the presence of two or more different heterocyclic moieties in a single molecule often enhances the biocidal profile remarkably, we investigated the sonocatalytic chemoselective synthesis of pyrazolo[3,4-b]pyridine derivatives using NaCl as a catalyst in aqueous medium (Scheme 1).
2. Results and discussion
Initially, in order to optimize the reaction conditions, we choose the reaction of 4-chlorobenzaldehyde 1a, ethyl cyanoacetate 2 and 3-amino-5-methylpyrazol 3 as a model reaction. The effect of various solvents on the model reaction was conducted in the presence of NaCl under ultrasound irradiation (Table 1). The results indicated that the solvents had a significant effect on the product yield. The use of toluene, THF and CH2Cl2 as solvent gave poor yields (Table 1, entry 4, 5 and 6). Solvents like EtOH, MeOH and CH3CN gave moderate yields (Table 1, Entries 1, 2 and 3). The best conversion was observed when the reaction was performed in water (Table 1, entry 7). Based on these results, water was then selected as the solvent for further investigations.| Entry | Solvent | Time (min) | Yield* (%) |
|---|---|---|---|
| a Reactions are performed on a 2 mmol scale of the reactants and 10 mol% of NaCl under ultrasonic irradiation. * Isolated yield. | |||
| 1 | Ethanol | 55 | 79 |
| 2 | Methanol | 70 | 74 |
| 3 | Acetonitrile | 50 | 68 |
| 4 | Toluene | 120 | 47 |
| 5 | THF | 120 | 49 |
| 6 | CH2Cl2 | 105 | 51 |
| 7 | Water | 25 | 94 |
Several variables may affect the efficiency of ultrasonication, in particular the increase in the viscosity of the reaction medium decreases the efficiency of ultrasound. Indeed, the ultrasonic waves became unable to overcome the cohesive forces of very strong mix. Indeed, the size of the micro spheres becomes very small, which significantly influence the reaction yield. The increase of the yield in sonication can be explained by the decrease of the viscosity of reaction mixture, which allows the acceleration of the molecular diffusion of the reagents which in turn increases the number of ultrasonic cavitation bubbles per unit volume.19
To find the specific effect of ultrasound on this reaction, the above mentioned reaction was carried out under conventional heating. There is no product formation in the absence of catalyst even after refluxing for 24 h (Table 2, entry 1) but product 4b was obtained after 150 min under ultrasound irradiation in moderate yield in absence of a catalyst (Table 2, entry 1). The dramatic increase in reaction rate under ultrasound irradiation could be ascribed to the simultaneous intensive enhancements of both heat and mass transfer.20 It is presumed that the efficiency using ultrasound irradiation is due to the cavitation phenomena. The chemical and physical effects of ultrasound arise from the cavitational collapse which produce extreme conditions locally and thus induce the formation of chemical species not easily attained under conventional conditions.21,22 However the results demonstrated the need of a catalyst. Thus in order to develop a feasible approach, a comparison of efficiency of catalytic activity of NaCl with several catalysts is presented in Table 2. Result showed that NaCl is superior to the other catalysts in terms of yield and reaction time and exhibit 15 fold higher TOF value as compared to conventional heating. The hydrophobic effect23 of water (all reactants due to their dislike for water come close enough to react) is further increased by presence of NaCl due to the salting-out effect.24 The salting-out effect of NaCl is quite well understood.25 When NaCl dissolve in water, there is a volume contraction, electrostriction, as water collapses around the ions to solvate it, which decreases the number of water molecules available to interact with the reactants. As a result of the increasing demand of solvent molecules, the reactant–reactant interactions are stronger than the solvent–reactant interactions; the reactant molecules react by forming hydrophobic interactions with each other. This salting-out effect is more pronounced for NaCl than for KCl,26 which shows the superiority of NaCl as a catalyst.
| Entry | Catalyst | Ultrasonic irradiation | Conventional condition | ||||
|---|---|---|---|---|---|---|---|
| Time (min) | Yield* (%) | TOF (h−1) | Time (h) | Yield* (%) | TOF (h−1) | ||
| a Reactions are performed on a 2 mmol scale of the reactants and 10 mol% of catalyst. NR = no reaction. * Isolated yield. | |||||||
| 1 | — | 150 | 35 | — | 24 | NR | — |
| 2 | Sulfamic acid | 110 | 54 | 44 | 20 | 62 | 5 |
| 3 | InCl3 | 80 | 71 | 80 | 12 | 69 | 9 |
| 4 | In(OTf)3 | 75 | 79 | 95 | 11 | 72 | 10 |
| 5 | CAN | 60 | 82 | 123 | 8 | 74 | 14 |
| 6 | NaCl | 25 | 94 | 338 | 5 | 78 | 23 |
| 7 | LiCl | 25 | 68 | 245 | 5 | 51 | 15 |
| 8 | KCl | 25 | 79 | 284 | 5 | 64 | 19 |
| 9 | NaBr | 25 | 72 | 259 | 5 | 57 | 17 |
| 10 | NaI | 25 | 67 | 241 | 5 | 52 | 16 |
It is worthy to note that, ultrasound with frequencies less than 50 kHz and presence of NaCl in the reaction mixture has resulted in increase in the reaction rate when compared to the silent reaction because of the local rise in the temperature and pressure, accelerated solvolysis27 process of NaCl, which also increases the hydrophobic effect of water. Therefore, NaCl concentration forces a biphasic suspension (water and organic reactants) in which the reactants undergo an intimate ultrasound promoted mixing thereby increased reaction rate was observed as compared to conventional method. Coupling of these can offer an extraordinary synergistic effect with greater prospectives than these components in seclusion.
In order to optimize the amount of NaCl for the synthesis of the target compounds, we started the study by treating a mixture of 4-chlorobenzaldehyde 1a, ethyl cyanoacetate 2 and 3-amino-5-methylpyrazol 3 in the presence of different amounts of NaCl in water under ultrasonic irradiation to get the desired product. The results of this study are summarized in Table 3. It is noted that, 10 mol% of NaCl gave the best result in terms of time of completion and the product was obtained in 94% yield (Table 3, entry 3).
| Entry | NaCl (mol%) | Time (min) | Yield* (%) | TOF (h−1) |
|---|---|---|---|---|
| a Reactions are performed on a 2 mmol scale of the reactants under ultrasonic irradiation. * Isolated yield. | ||||
| 1 | 00 | 150 | 35 | — |
| 2 | 5 | 55 | 67 | 196 |
| 3 | 10 | 25 | 94 | 338 |
| 4 | 15 | 25 | 93 | 211 |
We also observed the effect of frequency of ultrasound irradiation on the reaction. When the frequency was 25 kHz, the model reaction gave the desired product 4b in 94% yield at 25 °C. Using 40 kHz did not change reaction yield a considerable amount (92% in the similar time). Experiments performed at constant transmitted power but variable frequency (25 and 40 kHz) shows the same trend (Table 4, entries 1 and 2). It is shown that there is an optimum frequency for effective synthesis of 4b in the frequency of 25 kHz. In order to further improve the yield of the reaction, we tried to perform three experiments in 30, 50, and 60 °C under ultrasonic irradiation (Table 4). We found that high temperature (50 °C) could improve the reaction yield and shorten the reaction time. But the reaction got the similar yield at 60 °C. Thus, we selected the water as solvent under ultrasound irradiation conditions for the one-pot reaction of aldehyde, ethyl cyanoacetate and 3-amino-5-methylpyrazole to give corresponding pyrazolo[3,4-b] pyridine-5-carbonitriles derivatives at 50 °C in the frequency of 25 kHz.
| Entry | Frequency (kHz) | Temp. (°C) | Time (min) | Yield* (%) |
|---|---|---|---|---|
| a Reactions are performed on a 2 mmol scale of the reactants under ultrasonic irradiation in water at 50 °C in the frequency of 25 kHz. * Isolated yield. | ||||
| 1 | 25 | 25 | 40 | 87 |
| 2 | 40 | 25 | 45 | 85 |
| 3 | 25 | 50 | 25 | 94 |
| 4 | 25 | 60 | 30 | 94 |
Literature reveals that the multicomponent condensation reactions of 5-aminopyrazoles with active methylene compounds and aromatic aldehydes, is accompanied by the occurrence of dehydrogenated pyrazolopyridines as side products,28 and sometimes dehydrogenated pyrazolopyridines, bis-pyrazolopyridines and pyrazolo[1,5-a]pyrimidine derivatives have been isolated as the main product28–30 instance of pyrazolo[3,4-b]pyridines due to the presence of nonequivalent nucleophilic reaction centers in the aminopyrazole building block.31,32 The present protocol gives pyrazolo[3,4-b]pyridine derivatives selectively (Scheme 1).
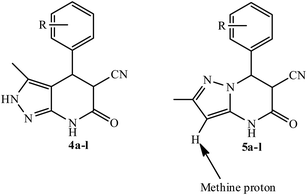
The excellent chemoselectivity observed between two nucleophilic centre of 3-amino-5-methylpyrazole i.e. one NH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and other is NH2–C–CH. Thus, when 3-amino-5-methylpyrazole was treated with benzaldehydes and ethyl cyanoacetate, the corresponding pyrazolo[3,4-b]pyridine derivatives 4a–l were formed exclusively. The proton NMR spectrum lacked characteristic singlet30 for the pyrazole methine near 6.0 ppm. This revealed that the reaction occurred through NH2–C
N and other is NH2–C–CH. Thus, when 3-amino-5-methylpyrazole was treated with benzaldehydes and ethyl cyanoacetate, the corresponding pyrazolo[3,4-b]pyridine derivatives 4a–l were formed exclusively. The proton NMR spectrum lacked characteristic singlet30 for the pyrazole methine near 6.0 ppm. This revealed that the reaction occurred through NH2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N nucleophilic centre and cyclisation mode lead to pyrazolo[3,4-b]pyridine 4a–l and ruled out the possibility of formation of pyrazolo[1,5-a]pyrimidine alternative 5a–l (Fig. 1).
N nucleophilic centre and cyclisation mode lead to pyrazolo[3,4-b]pyridine 4a–l and ruled out the possibility of formation of pyrazolo[1,5-a]pyrimidine alternative 5a–l (Fig. 1).
The general efficiency of this protocol was then studied for the synthesis of a variety of pyrazolo[3,4-b]pyridine-5-carbonitrile derivatives and the results are summarized in Table 5. A series of aromatic aldehydes with both electron-donating and electron-withdrawing substituents were reacted with ethyl cyanoacetate and 3-amino-5-methylpyrazol under the optimized reaction conditions. As shown in Table 5, all reactions went on smoothly and quickly under ultrasonic irradiation. The results indicated that variation in the yields is very little for both electron-rich and electron-deficient aldehydes. The reactions proceeded to completion in short durations, and the pure products were obtained simply by recrystallization from ethanol without involving any chromatographic purification.
| S. No | R | Time (min) | Product | Yield* (%) | trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratio cis ratio |
Mp (°C) |
|---|---|---|---|---|---|---|
| a * Isolated yield. | ||||||
| 1 | H | 29 |  |
89 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
294–296 |
| 2 | 4-Cl | 25 | 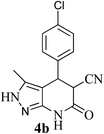 |
94 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 43 |
>340 |
| 3 | 4-Br | 27 | 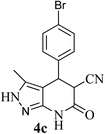 |
93 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
334–336 |
| 4 | 4-CH3 | 26 | 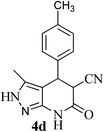 |
94 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 47 |
316–318 |
| 5 | 4-OCH3 | 27 | 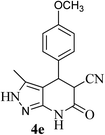 |
92 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
322–324 |
| 6 | 4-F | 23 | 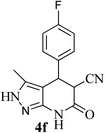 |
95 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
272–274 |
| 7 | 4-OH | 24 | 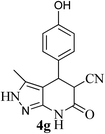 |
94 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
326–328 |
| 8 | 2,6-Cl | 22 | 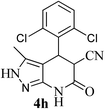 |
95 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
>340 |
| 9 | 2-F-6-Cl | 20 | 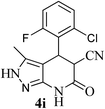 |
95 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
302–304 |
| 10 | 3,4,5-OCH3 | 24 | 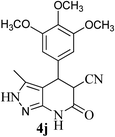 |
92 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 36 |
270–272 |
| 11 | 3-OPh | 33 | 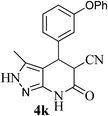 |
84 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 41 |
181–183 |
| 12 | — | 21 | 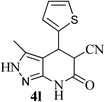 |
92 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57 57 |
264–266 |
The 1H NMR spectra of the products indicated the formation of two diastereoisomers (cis and trans). The 1H NMR spectrum of a mixture of cis- and trans isomer of 4b is presented in Fig. 2. We found that trans isomer was formed in more ratio compared to cis isomer while benzaldehydes with electron-withdrawing substituents produced a slightly higher ratio of trans products. Surprisingly it was found that cis isomer of 4l was formed in higher ratio as compared to trans isomer (Table 4, entry 12).
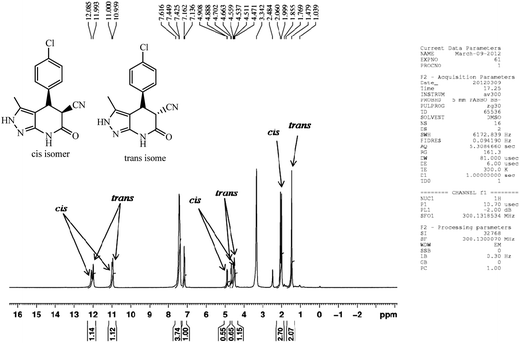 | ||
| Fig. 2 The 1H NMR signals of the cis and trans isomers of 4-(4-chlorophenyl)-3-methyl-6-oxo-4,5,6,7-tetrahydro-2H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4b). | ||
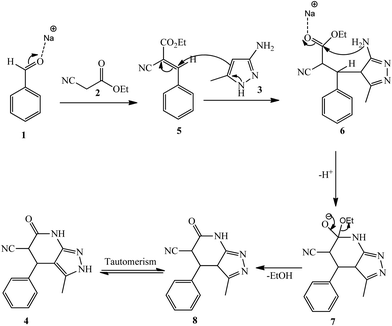
Mechanistically, the formation of compound 4 could be explained by the reaction sequence in Scheme 2. First, a Knoevenagel condensation reaction of aldehyde 1 with α-cyano ethylacetate 2 is proposed to give the intermediate 5 then Michael addition of electron-rich amino heterocycle 3 to 5 should have taken place to provide intermediate 6, which in turn undergoes intramolecular cyclization resulting ring system 7, subsequently loss of EtOH formed 8. Finally, compound 8 undergoes tautomerism to produce 3-methyl-6-oxo-4-phenyl-4,5,6,7-tetrahydro-2H-pyrazolo[3,4-b]pyridine-5-carbonitrile 4b, respectively.
3. Experimental
Melting points were recorded on a Toshniwal apparatus and are uncorrected. The purity of compounds was checked on thin layers of silica gel in various non-aqueous solvent systems e.g. n-hexane–ethyl acetate, (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), benzene–ethyl acetate (9
3), benzene–ethyl acetate (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), benzene–dichloromethane (8
1), benzene–dichloromethane (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). IR spectra (KBr) were recorded on a Shimadzu FT IR-8400s spectrophotometer and 1HNMR and 13CNMR spectra were recorded on a Bruker DRX-300 instrument at 300 and 75 MHz, respectively, in DMSO-d6 relative to tetramethylsilane as an internal reference. Mass spectrum of representative compound was recorded on Waters Xevo Q-Tof and API-2000 AB SCIEX spectrometer at 70 eV. Ultrasound irradiation was provided by ultrasonic processor probe (Processor SONOPROS PR-1000MP, OSCAR ULTRASONICS with power input 230 V, 50 Hz, 4 A and power variac 0–230 V and 3 A) operating at 20 kHz, 750 W with 6 mm/12 mm tip diameter probe.
2). IR spectra (KBr) were recorded on a Shimadzu FT IR-8400s spectrophotometer and 1HNMR and 13CNMR spectra were recorded on a Bruker DRX-300 instrument at 300 and 75 MHz, respectively, in DMSO-d6 relative to tetramethylsilane as an internal reference. Mass spectrum of representative compound was recorded on Waters Xevo Q-Tof and API-2000 AB SCIEX spectrometer at 70 eV. Ultrasound irradiation was provided by ultrasonic processor probe (Processor SONOPROS PR-1000MP, OSCAR ULTRASONICS with power input 230 V, 50 Hz, 4 A and power variac 0–230 V and 3 A) operating at 20 kHz, 750 W with 6 mm/12 mm tip diameter probe.
3.1 Conventional synthesis of 4-(4-chlorophenyl)-3-methyl-6-oxo-4,5,6,7-tetrahydro-2H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4b)
A mixture of 4-chlorobenzaldehyde 1b (2.0 mmol), ethyl cyanoacetate 2 (2.0 mmol) and 3-amino-5-methylpyrazole 3 (2.0 mmol) and 10 mol% sodium chloride was refluxed in water (30 ml) for 5 h. After completion of the reaction as indicated by TLC (n-hexane–ethyl acetate, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), the reaction mixture was allowed to cool. The solid product was filtered and washed with water, dried and recrystallised from ethanol.
3), the reaction mixture was allowed to cool. The solid product was filtered and washed with water, dried and recrystallised from ethanol.
3.2 Ultrasonics synthesis of 4-(4-chlorophenyl)-3-methyl-6-oxo-4,5,6,7-tetrahydro-2H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4b)
A mixture of 4-chlorobenzaldehyde 1b (2.0 mmol), ethyl cyanoacetate 2 (2.0 mmol) and 3-amino-5-methylpyrazole 3 (2.0 mmol) and 10 mol% sodium chloride in 20 ml water was introduced in a heavy walled pear-shaped two-necked flask with non-standard taper outer joint. The flask was attached to a 12 mm tip diameter probe and the reaction mixture was sonicated for the 25 min at 50% power of the processor and in a 4 s pulse mode till a solid product separates out. Completion of the reaction was monitored by TLC using n-hexane–ethyl acetate (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as the eluent. Upon completion of the reaction, the solid product was filtered and washed with water, dried and recrystallised from ethanol.
3) as the eluent. Upon completion of the reaction, the solid product was filtered and washed with water, dried and recrystallised from ethanol.
4. Conclusion
In summary, ultrasound has accelerated the three-component reaction of 3-amino-5-methylpyrazole, ethyl cyanoacetate, and aromatic aldehydes in the presence of catalytic amounts of NaCl. The chemoselective synthesis of pyrazolo[3,4-b]pyridine by this new method has proved to be useful both from economical and environmental points of view. About 15-fold higher catalytic activity was observed for NaCl under ultrasonic irradiation than that under the conventional method. The results suggested that ultrasonic irradiation and NaCl had a synergistic effect. Good functional group tolerance, broad scope of usable substrates, excellent yields and short reaction time are prominent features of the present sonocatalyzed methodology. This methodology also overcomes the formation of unwanted by-products, low yields and external high temperatures. We expect this method will find extensive applications in the field of combinatorial chemistry, diversity-oriented synthesis, sonochemistry, and drug discovery. All compounds were synthesized in good to excellent yield and the spectral analysis confirmed their structures.Acknowledgements
Financial assistance from the CSIR [02(0143)/13/EMR-II], New Delhi is gratefully acknowledged. We are also thankful to the Central Drug Research Institute (CDRI), Lucknow and IIT Delhi for the spectral analyses.References
- (a) P. Cintas and J. L. Luche, Green Chem., 1999, 1, 115 RSC; (b) R. B. Nasir Baig and R. S. Varma, Chem. Soc. Rev., 2012, 41, 1559 RSC; (c) K. J. Jarag, D. V. Pinjari, A. B. Pandit and G. S. Shankarling, Ultrason. Sonochem., 2011, 18, 617 CrossRef CAS PubMed.
- (a) Ultrasound: Its Chemical, Physical, and Biological Effects, ed. K. S. Suslick, VCH, New York, 1988 Search PubMed; (b) B. S. Singh, H. R. Lobo, D. V. Pinjari, K. J. Jarag, A. B. Pandit and G. S. Shankarling, Ultrason. Sonochem., 2013, 20, 633 CrossRef CAS PubMed.
- (a) J. T. Li, Y. Yin and M. X. Sun, Ultrason. Sonochem., 2010, 17, 363 CrossRef CAS PubMed; (b) J. T. Li, S. X. Wang, G. F. Chen and T. S. Li, Curr. Org. Synth., 2005, 2, 415 CrossRef CAS; (c) M. Mamaghani and S. Dastmard, Ultrason. Sonochem., 2009, 16, 445 CrossRef CAS PubMed; (d) T. S. Saleh and N. M. A. El-Rahman, Ultrason. Sonochem., 2009, 16, 237 CrossRef CAS PubMed.
- R. Cella and H. A. Stefani, Tetrahedron, 2009, 65, 2619 CrossRef CAS PubMed.
- (a) B. Ganem, Acc. Chem. Res., 2009, 42, 463 CrossRef CAS PubMed; (b) A. Padwa, Chem. Soc. Rev., 2009, 38, 3072 RSC; (c) L. Banfi, A. Basso, L. Giardini, R. Riva, V. Rocca and G. Guanti, Eur. J. Org. Chem., 2011, 100 CrossRef CAS.
- (a) A. Domling, Chem. Rev., 2006, 106, 17 CrossRef PubMed; (b) D. M. D'Souza and T. J. J. Muller, Chem. Soc. Rev., 2007, 36, 1095 RSC; (c) M. S. Singh and S. Chowdhury, RSC Adv., 2012, 2, 4547 RSC; (d) D. Tejedor and F. Garcia-Tellado, Chem. Soc. Rev., 2007, 36, 484 RSC; (e) V. Polshettiwar and R. S. Varma, Chem. Soc. Rev., 2008, 37, 1546 RSC.
- (a) C. J. Li and L. Chen, Chem. Soc. Rev., 2006, 35, 68 RSC; (b) C. J. Li, Chem. Rev., 2005, 105, 3095 CrossRef CAS PubMed; (c) G. Shore, W. J. Yoo, C. J. Li and M. Organ, Chem. – Eur. J., 2010, 16, 126 CrossRef CAS PubMed.
- (a) K. S. Gudmundsson, B. A. Johns and S. H. Allen, Bioorg. Med. Chem. Lett., 2008, 18, 1157 CrossRef CAS PubMed; (b) W. Schwede, H. Briem, H. Kuenzer, M. Husemann, G. Kettschau, M. Schaefer, A. Ter Laak, K.-H. Thierauch and S. J. Ince, US Pat. 7 842 809, 2010; (c) A. Feurer, J. Luithle, S. Wirtz, G. Koenig, J. Stasch, E. Stahl, R. Schreiber, F. Wunder and D. Lang, PCT Int. Appl., WO 2004009589; (d) S. A. Saggar, J. T. Sisko, T. J. Tucker, R. M. Tynebor, D. S. Su and N. J. Anthony, US Pat. Appl., US 2007/021442A1, 2007; (e) M. Cheung, P. A. Harris, J. G. Badiang, G. E. Peckham, S. D. Chamberlain, M. J. Alberti, D. K. Jung, S. S. Harris, N. H. Bramson, A. H. Epperly, S. A. Stimpson and M. R. Peel, Bioorg. Med. Chem. Lett., 2008, 18, 5428 CrossRef CAS PubMed.
- B. E. Dress, L. Chakravarty, G. D. Prestwich, G. Dorman, M. Kavecz, A. Lukacs, L. Urge, F. Darvas, P. W. Rzepecki and C. G. Ferguson, WO Pat. 016 245, 2005, Chem. Abstr., 2005, 142, 261534.
- D. W. S. Kung and G. Fuller, US Pat. 266 815, 2004, Chem. Abstr., 2005, 142, 266815.
- D. R. Bristow and I. L. Martin, J. Neurochem., 1990, 54, 751 CrossRef CAS.
- J. Zezula, A. Slany and W. Sieghart, Eur. J. Pharmacol., 1996, 301, 207 CrossRef CAS.
- (a) J. B. Patel and J. B. Malick, Eur. J. Pharmacol., 1982, 78, 323 CrossRef CAS; (b) S. A. Thompson, P. B. Wingrove, L. Connelly, P. J. Whiting and K. A. Wafford, Mol. Pharmacol., 2002, 61, 861 CrossRef CAS.
- D. B. Kendre, R. B. Toche and M. N. Jachak, Tetrahedron, 2007, 63, 11000 CrossRef CAS PubMed.
- (a) K. Balamurugan, S. Perumal and J. C. Menéndez, Tetrahedron, 2011, 67, 3201 CrossRef CAS PubMed; (b) J. Quiroga, S. Portillo, A. Pérez, J. Gálvez, R. Abonia and B. Insuasty, Tetrahedron Lett., 2011, 52, 2664 CrossRef CAS PubMed; (c) J. Quiroga, J. Trilleras, D. Pantoja, R. Abonía, B. Insuasty, M. Nogueras and J. Cobo, Tetrahedron Lett., 2010, 51, 4717 CrossRef CAS PubMed; (d) A. Rahmati, Tetrahedron Lett., 2010, 51, 2967 CrossRef CAS PubMed; (e) J. Quiroga, S. Cruz, B. Insuasty, R. Abonia, M. Nogueras and J. Cobo, Tetrahedron Lett., 2006, 47, 27 CrossRef CAS PubMed; (f) H. Zhibin, H. Yu, Z. Yao and S. Daqing, ACS Comb. Sci., 2011, 13, 45 CrossRef PubMed.
- (a) A. Dandia, A. K. Laxkar and R. Singh, Tetrahedron Lett., 2012, 53, 3012 CrossRef CAS PubMed; (b) M. A. Kolosov, V. D. Orlov, D. A. Beloborodov and V. V. Dotsenko, Mol. Diversity, 2009, 13, 5 CrossRef CAS PubMed.
- (a) A. Dandia, V. Parewa, A. K. Jain and K. S. Rathore, Green Chem., 2011, 13, 2135 RSC; (b) A. Dandia, R. Singh and S. Bhaskaran, Ultrason. Sonochem., 2011, 18, 1113 CrossRef CAS PubMed; (c) A. Dandia, D. S. Bhati, A. K. Jain and G. N. Sharma, Ultrason. Sonochem., 2011, 18, 1143 CrossRef CAS PubMed; (d) A. Dandia, R. Singh and S. Bhaskaran, Ultrason. Sonochem., 2010, 17, 399 CrossRef CAS PubMed.
- (a) A. Dandia, V. Parewa, S. L. Gupta and K. S. Rathore, J. Mol. Catal. A: Chem., 2013, 373, 61 CrossRef CAS PubMed; (b) A. Dandia, A. K. Jain, A. K. Laxkar and D. S. Bhati, Tetrahedron, 2013, 69, 2062 CrossRef CAS PubMed; (c) A. Dandia, V. Parewa and K. S. Rathore, Catal. Commun., 2012, 28, 90 CrossRef CAS PubMed; (d) A. Dandia, A. K. Jain and S. Sharma, Tetrahedron Lett., 2012, 53, 5859 CrossRef CAS PubMed; (e) A. Dandia, R. Singh, S. Bhaskaran and S. D. Samant, Green Chem., 2011, 13, 1852 RSC.
- J. Raso, P. Manas, R. Pagan and F. J. Sala, Ultrason. Sonochem., 1999, 5, 157 CrossRef CAS.
- (a) T. J. Mason, Chem. Soc. Rev., 1997, 26, 443 RSC; (b) G. J. Price, in Current Trends in Sonochemistry, ed. G. J. Price, Royal Society of Chemistry, Cambridge, 1992, pp. 87–109 Search PubMed.
- (a) G. Cravotto, E. C. Gaudinoa and P. Cintas, Chem. Soc. Rev., 2013, 42, 7521 RSC; (b) G. Cravotto and P. Cintas, Chem. Soc. Rev., 2006, 35, 180 RSC.
- (a) J. L. Luche, in Synthetic Organic Sonochemistry, Plenum Press, New York, 1998 Search PubMed; (b) M. Chtourou, R. Abdelhédi, M. H. Frikha and M. Trabelsi, Ultrason. Sonochem., 2010, 17, 246 CrossRef CAS PubMed.
- R. Breslow, Acc. Chem. Res., 1991, 24, 159 CrossRef CAS.
- F. A. Long and W. F. McDevit, Chem. Rev., 1952, 51, 119 CrossRef CAS.
- S. Endo, A. Pfennigsdorff and K. Goss, Environ. Sci. Technol., 2012, 46, 1496 CrossRef CAS PubMed.
- A. Hasseine, A.-H. Meniai and M. Korichi, Desalination, 2009, 242, 264 CrossRef CAS PubMed.
- (a) T. J. Mason, J. P. Lorimer and B. P. Mistry, Tetrahedron, 1985, 41, 5201 CrossRef CAS; (b) A. Tuulmets, Ultrason. Sonochem., 1997, 4, 189 CrossRef CAS.
- J. Quiroga, J. Portilla, H. Serrano, R. Abonia, B. Insuasty, M. Nogueras and J. Cobo, Tetrahedron Lett., 2007, 48, 1987 CrossRef CAS PubMed.
- X. Y. Zhang, X. Y. Li, X. S. Fan, X. Wang, J. J. Wang and G. R. Qu, Chin. Chem. Lett., 2008, 19, 153 CrossRef CAS PubMed.
- (a) J. Svetlik, L. Veizerová, T. U. Mayer and M. Catarinella, Bioorg. Med. Chem. Lett., 2010, 20, 4073 CrossRef CAS PubMed; (b) J. Quiroga, D. Mejia, B. Insuasty, R. Abonia, M. Nogueras, A. Sanchez, J. Cobo and J. N. Low, Tetrahedron, 2001, 57, 6947 CrossRef CAS; (c) J. Quiroga, B. Insuasty, R. Rincon, M. Larrahonda, N. Hanold and H. Meier, J. Heterocycl. Chem., 1994, 31, 1333 CrossRef CAS.
- (a) I. Drizin, R. J. Altenbach, S. A. Buckner, K. L. Whiteaker, V. E. Scott, J. F. Darbyshire, V. Jayanti, R. F. Henry, M. J. Coghlan, M. Gopalakrishnan and W. A. Carroll, Bioorg. Med. Chem., 2004, 12, 1895 CrossRef CAS PubMed; (b) J. Quiroga, B. Insuasty, A. Hormaza, C. Saitz and C. Jullian, J. Heterocycl. Chem., 1998, 35, 575 CrossRef CAS; (c) I. Drizin, M. W. Holladay, L. Yi, G. Q. Zhang, S. Gopalakrishnan, M. Gopalakrishnan, K. L. Whiteaker, S. A. Buckner, J. P. Sullivan and W. A. Carroll, Bioorg. Med. Chem. Lett., 2002, 12, 1481 CrossRef CAS.
- V. A. Chebanov, V. E. Saraev, S. M. Desenko, V. N. Chernenko, S. V. Shishkina, O. V. Shishkin, K. M. Kobzar and C. O. Kappe, Org. Lett., 2007, 9, 1691 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47231k |
| This journal is © The Royal Society of Chemistry 2014 |