Highly efficient [3 + 2] reaction of 3-vinylindoles with 3-indolylmethanols by Brønsted-acid catalysis†
Cheng Zhang,
Lan-Xi Zhang,
Yang Qiu,
Biao Xu,
Yu Zong and
Qi-Xiang Guo*
School of Chemistry and Chemical Engineering, Southwest University, Beibei, Chongqing 400715, China. E-mail: qxguo@swu.edu.cn
First published on 7th January 2014
Abstract
This paper describes a highly efficient [3 + 2] reaction between 3-vinylindoles and 3-indolylmethanols. Novel 2,3-bisindolylmethanes were prepared as single diastereoisomers in high yields with the promotion of 1 mol% 2-hydroxy-3,5-dinitrobenzoic acid.
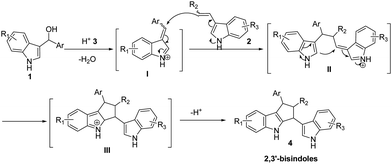
Bisindolylmethanes (BIMs) are molecules containing two indolyl moieties connected to the same carbon atom.1 These compounds mainly include 3,3′-BIMs, 2,2′-BIMs and 2,3′-BIMs, and are widely isolated from terrestrial and marine natural sources.2 Many of them have extensive biological and pharmaceutical activities.3 So, the synthesis of BIMs attracted extensive attention recently. Due to the high reactivity of the 3-position of indole, the 3,3′-BIMs could be easily prepared and numerous methods were developed for their synthesis.4 When the 3-position of indole is occupied, the 2-position can act as a nucleophilic site and afford 2,2′-BIMs.5 However, the synthesis of 2,3′-BIMs is much more difficult owing to the high reactivity of 3-position of indole.6 In fact, 2,3′-BIMs are of considerable value because they display a broad spectrum of interesting biological and pharmaceutical activities. For example, the natural product Yuehchukene, exhibiting anti-implantation activity, is a typical representation.7 So, chemists developed various methods to prepare Yuehchukene analogs and other 2,3′-BIMs. Recently, James McNulty and co-workers described a metal salt catalyzed dimerization of 3-vinylindoles.8 Inspired by this, and our previous works about using 3-indolylmethanols as alkylation reagents, we rationally designed a [3 + 2] reaction of 3-vinylindoles9 and 3-indolylmethanols10 to synthesize the 2,3-BIMs (Scheme 1). According to this rout, the 3-indolylmethanols 1 are dehydrated and produce the active vinylogous imino intermediates I in the promotion of an acid catalyst. Then, the 3-vinylindoles 2 will attack intermediate I to form a new active vinylogous imino intermediates II. An intramolecular Fridel–Crafts reaction of II will take place subsequently and the 2,3-bisindole compounds 4 will be formed. Here we reported this efficient and convenient method for the synthesis of 2,3-BIMs in high yields, excellent diastereoselectivities and with broad substrate scopes.
The 3-indolylmethanol 1a and trans-3-vinylindole 2a were chose as reactants in the model reaction. As expected, under the promotion of 10 mol% 3,5-dinitrobenzoic acid, the target product 4a was obtained in 80% yield (Table 1, entry 1). The crude 1H NMR analysis indicated that only one diastereoisomer was formed in this reaction. Encouraged by these good results, several substituted benzoic acids and other acids were screened. We found the acidity of Brønsted-acid was very important for this transformation. For example, the yield of 4a decreased greatly when we used 4-nitrobenzoic acid as catalyst (Table 1, entry 2), and only trace amount of product was obtained in the promotion of 4-methoxybenzoic acid (Table 1, entry 5). The strong Brønsted acids, such as 4-methylbenzenesulfonic acid (PTSA) and trifluoroacetic acid (TFA), also could promote this reaction, but the efficiency was not as well as that of 3,5-dinitrobenzoic acid (Table 1, entries 7, 8 vs. entry 1). In order to further increase the yield, the 2-hydroxy-3,5-dinitrobenzoic acid 3g was introduced in this reaction as a catalyst. Firstly, the 2-hydroxy-3,5-dinitrobenzoic acid 3g has the similar structure with 3,5-dinitrobenzoic acid. Moreover, the 2-hydroxy of 3g can increase the acidity of carboxyl through the formation of an intramolecular hydrogen bond.11 We found the 2-hydroxy-3,5-dinitrobenzoic acid 3g could promote this reaction efficiently in a short reaction time (Table 1, entry 9). Notably, the catalyst loading could be decreased to 1 mol% without any loss of the catalytic efficiency. This was sharply contrasted with the usual organocatalyed reactions in which 5–30 mol% organocatalyst was generally used.12 A clearer reaction was observed when this reaction was carried out at low temperature (Table 1, entry 10). This reaction stalled when the temperature was further lower (Table 1, entry 14). Based on these results, the optimal reaction condition for the [3 + 2] reaction between 3-indolylmethanol 1a and 3-vinylindole 2a was determined. Generally, this reaction was carried out in DCM at −20 °C by using 1 mol% 2-hydroxy-3,5-dinitrobenzoic acid 3g as the catalyst.
| Entry | 3 (x) | T (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a The reaction was carried out with 1a (0.1 mmol), 2a (0.1 mmol), 3 in solvent (1 mL).b Isolated yield. | ||||
| 1 | 3a (10) | 0 | 23 | 80 |
| 2 | 3b (10) | 0 | 138 | 30 |
| 3 | 3c (10) | 0 | 140 | 13 |
| 4 | 3d (10) | 0 | 155 | 22 |
| 5 | 3e (10) | 0 | 156 | 3 |
| 6 | 3f (10) | 0 | 92 | 84 |
| 7 | PTSA (10) | 0 | 22 | 62 |
| 8 | TFA (10) | 0 | 17 | 79 |
| 9 | 3g (10) | 0 | 5 | 86 |
| 10 | 3g (1) | 0 | 4 | 87 |
| 11 | 3g (1) | −10 | 3 | 93 |
| 12 | 3g (1) | −20 | 4 | 95 |
| 13 | 3g (1) | −30 | 23 | 95 |
| 14 | 3g (1) | −40 | 30 | 81 |
With the optimal reaction conditions in hand, we then explored the substrate scopes. Firstly, the substituents on the indole ring of 3-vinylindoles 2 were investigated (Table 2, entries 2–6). When electron-withdrawing groups were introduced on the indole ring of 2, the target products 4b–d were obtained as single diastereoisomers in excellent yields (Table 2, entries 2–4). It was found that the position of substituents has little effect on the diastereoselectivity and yield of this reaction. For example, the 4-chloro-3-styryl-1H-indole reacted with 1a under the optimal reaction conditions and produce the product 4b in a completely diastereo-controlling fashion and with quantitative yield (Table 1, entry 2); similar results were obtained when the position of substituents changed (Table 1, entries 3 and 4). Then, the 5-MeO and 6-Me indole substituted 3-vinylindoles were involved in this reaction. The desired products 4e and 4f were obtained in excellent yields (Table 2, entries 5 and 6). Secondly, the substituents effects on phenyl group of 3-indolylmethanols were studied. When electron-withdrawing groups were introduced into the phenyl ring, the reaction speed became slow (Table 2, entries 7–15). In particular, the nitro substituent decreased the reaction speed greatly (Table 2, entries 12–14). In these cases, the desired products 4l–m could be obtained in high yields when the reaction with a prolongation of reaction time (Table 2, entries 12–14). We found the reaction between (1H-indol-3-yl)(2-methoxyphenyl) methanol 1k and 2a produced the target product 4p in 56% yield, even the reaction time was prolonged to 119 hours (Table 2, entry 16). However, the 3-MeO phenyl substituted 1l produced the corresponding product in excellent yield (Table 2, entry 17). The steric influence of 2-MeO group of 1k is the possible reason that leads to the low yield of 4p. The relative structure of compound 4d was determined X-ray crystal analysis (Fig. 1).13 The relative structures of 4a–r were assigned correspondingly.
| Entry | 1 | 4 | Ar | R | T (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|---|
| a For entries 1–6: 1 (0.1 mmol), 2 (0.1 mmol), 3g (0.001 mmol) in CH2Cl2 (1 mL); entries 7–17: 1 (0.2 mmol), 2 (0.2 mmol), 3g (0.002 mmol) in CH2Cl2 (1 mL).b Isolated yield. | |||||||
| 1 | 1a | 4a | Ph | H | −20 | 4 | 95 |
| 2 | 1a | 4b | Ph | 4-Cl | −20 | 13 | 99 |
| 3 | 1a | 4c | Ph | 5-Br | −20 | 12 | 87 |
| 4 | 1a | 4d | Ph | 6-F | −20 | 18 | 86 |
| 5 | 1a | 4e | Ph | 5-MeO | −20 | 28 | 86 |
| 6 | 1a | 4f | Ph | 6-Me | −20 | 14 | 97 |
| 7 | 1b | 4g | 2-FC6H4 | H | 0 | 13 | 96 |
| 8 | 1c | 4h | 3-FC6H4 | H | 0 | 12 | 99 |
| 9 | 1d | 4i | 4-FC6H4 | H | 0 | 13 | 85 |
| 10 | 1e | 4j | 3,4-2ClC6H3 | H | 0 | 23 | 99 |
| 11 | 1f | 4k | 2-ClC6H4 | H | 0 | 14 | 92 |
| 12 | 1g | 4l | 2-NO2C6H4 | H | 0 | 89 | 79 |
| 13 | 1h | 4m | 3-NO2C6H4 | H | 0 | 113 | 93 |
| 14 | 1i | 4n | 4-NO2C6H4 | H | 0 | 113 | 98 |
| 15 | 1j | 4o | 4-CF3C6H4 | H | 0 | 22 | 91 |
| 16 | 1k | 4p | 2-MeOC6H4 | H | 0 | 119 | 56 |
| 17 | 1l | 4q | 3-MeOC6H4 | H | 0 | 17 | 99 |
It is well known that the BINOL-derived phosphoric acids have excellent catalytic and chiral induction abilities in organocatalysis.14,15 So, the phosphoric acid 3h was introduced to this reaction as a chiral Brønsted acid catalyst. We wanted to realize this [3 + 2] transformation in an asymmetric version. Disappointedly, the enantioselectivity of 4a was very poor (Table 3, entries 1–3). We thought the poor chiral induction maybe caused by the remote chiral-control, i.e. the reaction sites were far away from the chiral centre of catalysts (TS I).
| Entry | Time (h) | T (°C) | Yieldb (%) | eec (%) |
|---|---|---|---|---|
a The reaction was carried out with 1a (0.1 mmol), 2a (0.12 mmol), 3h (10 mol%) in CH2Cl2 (1 mL).b Isolated yield.c HPLC conditions: chiral Lux Cellulose-1 column, nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2-propanol = 90 2-propanol = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1 mL min−1, 30 °C, tminor = 31.836, tmajor = 35.743.d The reaction was carried out with 1a (0.12 mmol), 2a (0.1 mmol). 10, 1 mL min−1, 30 °C, tminor = 31.836, tmajor = 35.743.d The reaction was carried out with 1a (0.12 mmol), 2a (0.1 mmol). |
||||
| 1 | 2 | 25 | 84 | 8 |
| 2 | 5 | −10 | 75 | 10 |
| 3d | 5 | −30 | 60 | 5 |
In conclusion, we disclosed a highly efficient [3 + 2] reaction between 3-indolylmethanols and 3-vinylindoles. Under the promotion of 1 mol% 2-hydroxy-3,5-dinitrobenzoic acid 3g, the 2,3-BIMs 4a–q were prepared in high yields as single diastereoisomers.
We are grateful for financial support from NSFC (21272002), the Fundamental Research Funds for the Central Universities (XDJK2013B028) and the Program for New Century Excellent Talents in Universities (NCET-12-0929).
Notes and references
- M. Shiri, M. A. Zolfigol, H. G. Kruger and Z. Tanbakouchian, Chem. Rev., 2010, 110, 2250 CrossRef CAS PubMed.
- Selected examples: (a) E. Fahy, B. C. M. Potts, D. J. Faulkner and K. Smith, J. Nat. Prod., 1991, 54, 564 CrossRef CAS; (b) T. S. Kam, Alkaloids, Chemical and Biological Perspectives, Pergamon, Amsterdam, 1999 Search PubMed; (c) G. Bifulco, I. Bruno, L. Minale, R. Riccio, A. Calignano and C. Debitus, J. Nat. Prod., 1994, 57, 1294 CrossRef CAS PubMed; (d) G. Bifulco, I. Bruno, R. Riccio, J. Lavayre and G. Bourdy, J. Nat. Prod., 1995, 58, 1254 CrossRef CAS PubMed.
- See ref. 1 and the references cited in.
- Selected examples for the synthesis of 3,3′-BIMs: (a) T. V. Moskovkina, A. I. Kalinovskii, E. A. Martyyas and M. M. Anisimov, Chem. Heterocycl. Compd., 2013, 49, 452 CrossRef CAS; (b) G. S. S. Kumar, A. A. M. Prabu, S. J. Jenniefer, N. Bhuvanesh, P. T. Muthiah and S. Kumaresan, J. Mol. Struct., 2013, 1047, 109 CrossRef; (c) S. Gujarathi, H. P. Hendrichson and G. Zheng, Tetrahedron Lett., 2013, 54, 3550 CrossRef CAS; (d) S. B. Bharate, J. B. Bharate, S. I. Khan, B. L. Tekwani, M. R. Jacob, R. Mudududdla, R. R. Yadav, B. Singh, P. R. Shama, S. Maity, B. Singh, I. A. Khan and R. A. Vishwakarma, Eur. J. Med. Chem., 2013, 63, 435 CrossRef CAS PubMed; (e) K. Prinsen, M. M. Cona, J. Cleynhens, H. Vanbilloen, J. Li, N. Dyubankova, E. Lescrinier, G. Bormans, Y. Ni and A. Verbruggen, Bioorg. Med. Chem. Lett., 2013, 23, 3216 CrossRef CAS PubMed; (f) H. Veisi, M. Ataee, L. Fatolahi and S. Lotfi, Lett. Org. Chem., 2013, 10, 111 CrossRef CAS.
- Selected examples: (a) R. K. Sharma, Y. Monga and A. Puri, Catal. Commun., 2013, 35, 110 CrossRef CAS; (b) T. Nobuta, A. Fujiya, N. Tada, T. Miura and A. Itoh, Synlett, 2012, 23, 2975 CrossRef CAS; (c) P. Dydio, D. Lichosyt, T. Zieliński and J. Jurczak, Chem. – Eur. J., 2012, 18, 13686 CrossRef CAS PubMed; (d) P. J. Das and J. Das, Tetrahedron Lett., 2012, 53, 4718 CrossRef CAS.
- Selected examples: (a) T. Yokosaka, H. Nakayama, T. Nemoto and Y. Mamada, Org. Lett., 2013, 15, 2978 CrossRef CAS PubMed; (b) D. Fokas, M. Kaselj, Y. Isome and Z. Wang, ACS Comb. Sci., 2013, 15, 49 CrossRef CAS PubMed; (c) D. Shu, G. N. Winston-McPherson, W. Song and W. Tang, Org. Lett., 2013, 15, 4162 CrossRef CAS PubMed; (d) T. P. Pathak, J. G. Osiak, R. M. Vaden, B. E. Welm and M. S. Sigman, Tetrahedron, 2012, 68, 5203 CrossRef CAS PubMed.
- Selected examples for the synthesis of Yuehchukene: (a) K.-F. Cheng, Y.-C. Kong and T.-Y. Chan, J. Chem. Soc., Chem. Commun., 1985, 2, 48 RSC; (b) J.-H. Sheu, Y.-K. Chen, H.-F. Chung, S.-F. Lin and P.-J. Sung, J. Chem. Soc., Perkin Trans. 1, 1998, 12, 1959 RSC; for Yuehchukene anologies: (c) K.-F. Cheng, T.-T. Chan, T.-F. Lai and Y.-C. Kong, J. Chem. Soc., Perkin Trans. 1, 1988, 12, 3317 RSC; (d) K.-F. Cheng, G.-A. Cao, Y.-W. Yu and Y.-C. Kong, Synth. Commun., 1994, 24, 65 CrossRef CAS.
- J. McNulty and D. McLeod, Synlett, 2011, 5, 717 CrossRef.
- (a) C. Gioia, A. Hauville, L. Bernardi, F. Fini and A. Ricci, Angew. Chem., Int. Ed., 2008, 47, 9236 CrossRef CAS PubMed; (b) B. Tan, G. Hernandez-Torres and C. F. Barbas, III, J. Am. Chem. Soc., 2011, 133, 12354 CrossRef CAS PubMed; (c) M. Terada, K. Moriya, K. Kanomata and K. Sorimachi, Angew. Chem., Int. Ed., 2011, 50, 12586 CrossRef CAS PubMed; (d) G. Bergonzini, L. Gramigna, A. Mazzanti, M. Fochi, L. Bernardi and A. Ricci, Chem. Commun., 2010, 46, 327 RSC; (e) T. P. Pathak, J. G. Osiak, R. M. Vaden, B. E. Welm and M. S. Sigman, Tetrahedron, 2012, 26, 5203 CrossRef PubMed.
- (a) Q.-X. Guo, Y.-G. Peng, J.-W. Zhang, L. Song, Z. Feng and L.-Z. Gong, Org. Lett., 2009, 11, 4620 CrossRef CAS PubMed; (b) L. Song, Q.-X. Guo, X.-C. Li, J. Tian and Y.-G. Peng, Angew. Chem., Int. Ed., 2012, 51, 1899 CrossRef CAS PubMed; (c) B. Xu, Z.-L. Guo, W.-Y. Jin, Z.-P. Wang, Y.-G. Peng and Q.-X. Guo, Angew. Chem., Int. Ed., 2012, 51, 1059 CrossRef CAS PubMed; (d) J.-Z. Huang, S.-W. Luo and L.-Z. Gong, Acta Chim. Sin., 2013, 71, 879 CrossRef CAS; (e) J. Song, C. Guo, A. Adele, H. Yin and L.-Z. Gong, Chem. – Eur. J., 2013, 19, 3319 CrossRef CAS PubMed; (f) C. Guo, J. Song, J.-Z. Huang, P.-H. Chen, S.-W. Luo and L.-Z. Gong, Angew. Chem., Int. Ed., 2012, 51, 1046 CrossRef CAS PubMed.
- According to the SciFinder results, pKa3g = 1.57, pKa3a = 2.77.
- Selected examples: (a) H. Zhang, X. Wen, L. Gan and Y. Peng, Org. Lett., 2012, 14, 2126 CrossRef CAS PubMed; (b) Q.-X. Guo, H. Liu, C. Guo, S.-W. Luo, Y. Gu and L.-Z. Gong, J. Am. Chem. Soc., 2007, 129, 3790 CrossRef CAS PubMed.
- ESI.†.
- (a) T. Akiyama, Chem. Rev., 2007, 107, 5744 CrossRef CAS PubMed; (b) A. G. Doyle and E. N. Jacobsen, Chem. Rev., 2007, 107, 5713 CrossRef CAS PubMed; (c) M. Terada, Chem. Commun., 2008, 4097 RSC; (d) T. Akiyama, J. Itoh and K. Fuchibe, Adv. Synth. Catal., 2006, 348, 999 CrossRef CAS.
- For leading literature references, see: (a) D. Uraguchi and M. Terada, J. Am. Chem. Soc., 2004, 126, 5356 CrossRef CAS PubMed; (b) T. Akiyama, J. Itoh, K. Yokota and K. Fuchibe, Angew. Chem., Int. Ed., 2004, 43, 1566 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, structural proofs, CIF file of compound 4d. CCDC 968308. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ra47056c |
| This journal is © The Royal Society of Chemistry 2014 |