Structure, luminescence property and energy transfer behavior of color-adjustable La5Si2BO13:Ce3+,Mn2+ phosphors
Abstract
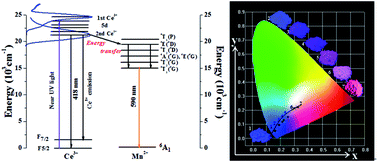
A series of color-adjustable phosphors La5Si2BO13(LSBO):Ce3+,Mn2+ were synthesized through a high temperature solid-state method. The crystal structures of Ce3+ and Mn2+ doped La5Si2BO13 phosphors were refined by the Rietveld method, which were proved to be the apatite-type hexagonal phase (space group of P63/m). It was found that two different La3+ sites in the La5Si2BO13 phase were occupied evenly by Ce3+ and Mn2+ ions, and then the formed vacancy contributed to the charge compensation. La5Si2BO13:Ce3+,Mn2+ phosphors exhibited a broad excitation band ranging from 250 to 375 nm and two broad emission bands centred at 418 nm and 585 nm upon 345 nm excitation. It is found that the emission colors could be tuned from blue-violet (0.1629, 0.0523) to pink (0.3227, 0.1830) by changing the ratio of Ce3+/Mn2+. Moreover, the energy transfer mechanism was verified to be the dipole–dipole interaction, and the critical distance was calculated to be 10.02 Å by using the concentration quenching method.

 Please wait while we load your content...
Please wait while we load your content...