Discovering p-doped mechanism in non-magnetic Ni–P films for HDD substrate: a combined experimental and theoretical study
Guofeng Cui*a,
Shaofang Liua,
Kaiming Wangb,
Qing Lic and
Gang Wuc
aKey Laboratory of Low-carbon Chemistry & Energy Conservation of Guangdong Province, School of Chemistry and Chemical Engineering, Sun-Yat Sen University, 510275, China. E-mail: cuigf@mail.sysu.edu.cn; Tel: +86-20-8411-0560
bSchool of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University, Shanghai, 200240, China
cMaterials Physics and Applications Division, Los Alamos National Laboratory, Los Alamos, New Mexico 87545, USA
First published on 3rd February 2014
Abstract
In this work, a new mechanism is proposed for the redox of hypophosphorous acid catalyzed by a nickel cluster through a combined study of density functional theory (DFT) calculations and electrochemical impedance spectroscopy (EIS) measurements. The DFT results indicate that the concentration of OH− is a crucial species to control the oxidation and reduction of hypophosphorous acid. The oxidation of hypophosphorous acid takes place preferably at higher OH− concentration, as OH− can combine directly with H3PO2 and hydrogen radical (H˙). In contrast, reduction is inhibited in this case because the hydrogen radical preferably combines with OH− rather than H3PO2. Thus, pH serves as a key switch to control the pathways of the coupling reaction. EIS results demonstrated that the electroless nickel process includes three electrochemical processes: charge–discharge of electrical double layer, Ni(I) transforming to Ni(II) or Ni(0), and specific adsorption of intermediate products. In good agreement with theoretical prediction, the experimental measurements indicated that an electroless nickel coating with high phosphorus content was successfully synthesized at a low pH, exhibiting non-magnetic properties and enabling its use as a non-magnetic coating for hard disk drive substrates.
1 Introduction
Electroless nickel (EN) deposits have been extensively used in the manufacturing of hard disk drives (HDD) substrates because they are able to exhibit non-magnetic characteristic when phosphorus content is higher than 20.0 at.%.1 Therefore, it is important to understand how to control effectively the phosphorus content in EN deposits.The EN process is a complicated heterogeneous catalytic process. It is well known that phosphorus doping in EN is derived from hypophosphite, a reducing agent for EN. During the deposition process, the redox of hypophosphite simultaneously occurs. In particular, the oxidation of hypophosphite leads to the formation of ortho-phosphite. The reduction of hypophosphite generates phosphorus, resulting in the doping of P into EN deposits. However, to date, the mechanism of the redox coupled reaction has not been fully understood, mainly due to the coupling caused by the shared hypophosphite reactant.
For the oxidation mechanism of hypophosphorous acid, there are two possible reaction pathways. One mechanism was proposed by Van den Meerakker.2 with hypophosphorous acid releasing directly one hydrogen radical. The other pathway was proposed by Homma,3,4 in which hypophosphorous acid should initially combine with one hydroxyl ion. Additionally, Homma calculated the relative energy for all possible intermediates (with DFT on the level of MP2/6-311G(d,p)) and the energy barrier of this reaction pathway is 423.4 kJ mol−1. However, the transition states and catalytic behavior of nickel species were neglected in the calculations. Compared to Meerakker's mechanism, the pathway of Homma is more possible according to our previous work.5 Here, we focus on the Homma pathway to elucidate further the reaction mechanism.
For the reduction mechanism of hypophosphorous acid, there are also two possible reaction mechanisms. One pathway is the direct phosphorus generation mechanism proposed by Brenner.6 He considered that hypophosphorous acid first loses two hydrogen atoms followed by the breaking of two P–O bonds. The other pathway is an indirect phosphorus generation mechanism proposed by Saitou,7–9 in which PH3 is considered to be a key intermediate. In this mechanism, H3PO2 firstly breaks two P–O bonds and then one hydrogen radical combines with a phosphorus atom in PH2 to form PH3. The two possible mechanisms are evidenced by the electrochemical measurement data during the electroplating of Ni–P alloy. In this work, we will analyze the feasibility of the double mechanism without considering the external electric field.
Many researchers have tried to investigate the mechanisms either by electrochemical10,11 or quantum chemical modeling methods.3,4,12,13 However, the results show that a single method cannot fully elucidate the coupled processes. In this work, we explore the double-reaction mechanism through a combination of electrochemical and quantum chemical modeling methods. The results provide new insights on how to control the pathway of the redox reaction of hypophosphorous acid and precisely predict the phosphorus content in the EN deposits.
Furthermore, in order to understand the coupled reaction pathways, the effect of OH− was investigated. During the EN process, different concentrations of OH− are found to be able to tune the reduction reaction of hypophosphite, and the OH− switching mechanism for this coupled reaction is proposed for the first time. The combination of DFT and EIS methods is proved to be an effective way to analyze the mechanism of this complicated electrochemical process.
2 Experimental and calculation methodology
2.1 EN Deposition on Al surface
Aluminum surfaces were polished by SiC with grade 600 and 1200 sandpapers. Then, they were washed with acetone. After the Al samples were rinsed, they were zinc immersion plated. First, the samples were immersed in a solution containing 30 g L−1 NiSO4·6H2O, 40 g L−1 ZnSO4·7H2O, 106 g L−1 NaOH, 10 g L−1 KCN, 5 g L−1 CuSO4·5H2O and 2 g L−1 FeCl3 at 25 ± 2 °C. After this first zinc immersion, the samples were rinsed by water. Subsequently, the zincate layer was stripped by a 50% nitric acid solution at 25 ± 2 °C for 20–30 s. Then, the samples were rinsed with water. Subsequently, the Al samples were immersed in the zincating immersion solution for a second time. After water rinsing, a uniform and fined zincate film was formed on the Al alloy sample surface.After the pretreatment, the samples were plated in electroless nickel solution. Electroless nickel solution was prepared with analytical grade reagents and Millipore ultrapure water with a resistance of 18 MΩ or higher. The solution contained 26 g L−1 nickel sulfate, 32 g L−1 sodium hypophosphite, 16 g L−1 sodium acetate, 4 g L−1 lactic acid and 8 g L−1 citrate acid. The electrolytes were heated to 82 ± 1 °C and purged by bubbling N2 before deposition. The pH value of the electrolyte was adjusted between 4.0 and 9.0 with KOH solution. The pH value was not adjusted again in the measurement process in order to avoid artificial interference. A detailed experimental method is given in our previous work.14,15
2.2 Structure and composition analysis
The chemical composition of Ni–P alloys was analyzed by X-ray fluorescence (XRF) spectrometer model S4-Explorer from Brukeraxs (Germany). The XRF spectra were evaluated by the automatic analysis program Spectra plus. Visible reflectance measurements on the EN surfaces were carried out using a 300 mm diameter integrating sphere and a 30 mW He–Ne laser operating at 633 nm. The magnetic properties of samples were analyzed by an MPMS XL-7 magnetic property measurement system (Quantum design, Inc.).2.3 DFT calculation method
All computations reported were carried out with the Gaussian 03 code,16 employing the hybrid Becke exchange and Lee, Yang, and Parr correlation (B3LYP)17–19 density functional method. For hydrogen, oxygen, chlorine and phosphorus, 6-311G(d,p) basis sets were used in the present work. The ECP (effective core potential) of the LANL2DZ basis set was adopted by Hay and Wadt for the nickel atoms.20 Those basis sets are effective on study of the mechanism of electroless13 and other electrochemical processes.21,22 Following these works, we have utilized these basis sets in this work. The molecular ground state geometry and vibrational frequency spectra of all species were calculated to determine the reaction pathway potential energy surface. Stationary points were determined with no imaginary components in the vibrational spectra, while transition states were determined with exactly one imaginary vibrational mode. Visual inspection of imaginary vibrational modes was performed with the Gaussian View 3.7 program package.The solvation effect of an aqueous solution was considered using an explicit solvating model. In the model, the Ni(II) ions complex with two water molecules. Differing from the previous model of Homma, our reaction model is a closed system with hypophosphite ions and Ni(II) ions simultaneously on the nickel cluster surface.
2.4 EIS measurement method
Electrochemical measurements were performed in a three-electrode cell. A 20 × 50 × 0.5 mm3 Al2O3 ceramic electrode sputtered by nickel coating was the working electrode. A 10 × 10 × 0.3 mm2 platinum foil and a saturated Hg/HgSO4 electrode (MSE) were used as the counter and reference electrodes, respectively. Prior to the measurements, the working electrode was cleaned with ethanol and acetone. The reference electrode was protected by a glass tube filled with saturated K2SO4 solution to avoid possible contamination.EIS was performed on an electrochemical workstation model ref. 600 (Gamry Inc.). An AC voltage (sinusoidal wave) with amplitude of 10.0 mV was used as the input signal, and the frequency range was set from 10 kHz to 0.5 mHz. A defined sample area of 1.0 cm2 was exposed to the electrolyte. A Luggin capillary was placed near the working electrode to minimize the ohmic drop. All electrode potentials are referred to saturated Hg/HgSO4 electrode (MSE), if not otherwise stated. All the EIS measurements were performed at the deposition potential Edep, with respect to the MSE reference electrode.
3 Results and discussion
3.1 Effect of phosphorus content on magnetic properties of EN films
The content of phosphorus in EN deposits obtained from different pH solutions is shown in Fig. 1. The incorporated phosphorus content gradually decreases from 13.8 wt% to 4.3 wt%, when pH values increase from 5.0 to 9.0. In particular, when the increment of pH values is 1.0, the corresponding phosphorus mass content decreases ca. 2.0 percent.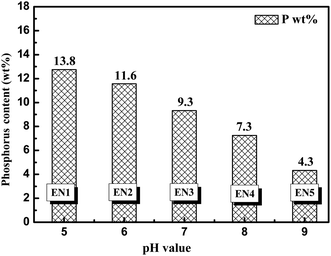 | ||
| Fig. 1 Phosphorus content (determined by XRF) in EN deposited Ni–P coatings as a function of pH values in solutions ranging from 5.0 to 9.0. | ||
The magnetic properties of EN deposits were measured as a function of phosphorus content of the EN samples (Fig. 2). Obviously, EN5 is magnetic in comparison with the other EN samples in Fig. 2a. The slopes of the line of magnetic force gradually decreased from EN4 to EN1, as shown in Fig. 2b. Additionally, the area of magnetic domain is also reduced as phosphorus content is increased, attributable to a phosphorus-interfaced magnetic domain. Furthermore, the structure of the EN film is changed from crystalline to amorphous, when the doped phosphorus content increases. A non-magnetic EN coating is essential when it is used as a substrate for HDDs without interference with the magnetic recording material. Thus, EN1with a higher phosphorus content film is more suitable as a HDD substrate.
 | ||
| Fig. 2 Magnetic properties of EN deposited Ni–P films (a and b) as a function of phosphorus content, and image of HDD disk obtained with EN1 conditions (c). | ||
3.2 Coupling reaction pathways from hypophosphorous acid
The coupled reaction pathways for hypophosphorous acid and Ni(II) compounds are illustrated in Scheme 1. The reaction pathways involve one oxidation pathway (OP) and three possible reduction pathways (DRP-I, DRP-II and IRP). As for the OP pathway, the [Ni–(H3PO2)]2+ compound first reacts with OH− to form IM1. Subsequently, the [Ni–(H4PO3)]+ decomposes and releases a hydrogen radical and one electron. This reaction pathway was first proposed by Homma.13 As for the DRP-I pathway, the [Ni–(H3PO2)]2+ compound releases two H˙ successively. Subsequently, the double bond in P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O turns into a single bond by hydrogenation of the oxygen atom. Finally, the [Ni–(H2PO2)]2+ (IM3) compound loses two OH−. During the DRP-II pathway, a hydrogen intermolecular transfer process occurs from the IM1 to the IM3 step, differentiating it from the DRP-I pathway. First, the [Ni–(H3PO2)]2+ compound releases one hydrogen radical. Next, the P
O turns into a single bond by hydrogenation of the oxygen atom. Finally, the [Ni–(H2PO2)]2+ (IM3) compound loses two OH−. During the DRP-II pathway, a hydrogen intermolecular transfer process occurs from the IM1 to the IM3 step, differentiating it from the DRP-I pathway. First, the [Ni–(H3PO2)]2+ compound releases one hydrogen radical. Next, the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond is broken via hydrogen intermolecular transfer from a phosphorus atom to an oxygen atom. Subsequently, the [Ni–(H2PO2)]2+ (IM3) compound releases two OH− and forms an adsorbed phosphorus atom. In the case of the IRP pathway, the P–O bond breaks before the scission of P–H bonds. The [Ni–(H3PO2)]2+ compound (R) turns into [Ni–(PH2)]2+ (IM3) accompanied by the loss of two OH. Subsequently, two hydrogen atoms are released as hydrogen radicals. As a result, the phosphorus atom is doped into the nickel coatings.
O double bond is broken via hydrogen intermolecular transfer from a phosphorus atom to an oxygen atom. Subsequently, the [Ni–(H2PO2)]2+ (IM3) compound releases two OH− and forms an adsorbed phosphorus atom. In the case of the IRP pathway, the P–O bond breaks before the scission of P–H bonds. The [Ni–(H3PO2)]2+ compound (R) turns into [Ni–(PH2)]2+ (IM3) accompanied by the loss of two OH. Subsequently, two hydrogen atoms are released as hydrogen radicals. As a result, the phosphorus atom is doped into the nickel coatings.
3.3 DFT analyze feasibility of reaction pathways
The oxidation pathway and potential energy profile of hypophosphorous acid via the OP pathway are illustrated in Scheme 1 and Fig. 3, respectively. During the OP pathway, the hypophosphorous acid combines with OH− and forms IM1 first. In this process, the relative energy of TS1 is 186.5 kJ mol−1, which is the highest energy barrier in the whole route. Subsequently, the IM1 compound loses one hydrogen radical and one electron. During the reaction from IM1 to the product, the energy barrier is 91.5 kJ mol−1. Obviously, the highest energy barrier is the formation of TS1, which is calculated to be 236.9 kJ mol−1 lower than that in Homma's model.4 The decreased energy barrier can be attributed to the catalysis of Ni(II) and the Ni9 cluster.During the formation of TS1, Ni(II) preferably attracts the electron cloud on oxygen in [Ni–(H3PO2)]2+. This makes the electropositivity of the phosphorus atom increase in the [Ni–(H3PO2)]2+ compound. Additionally, the higher electropositivity of the phosphorus atom is beneficial for combination with OH−. A detailed charge distribution of IM1 is given in Fig. 7a.
The direct reduction pathway of hypophosphorous acid via the DRP-I is illustrated in Scheme 1 and Fig. 4. At first, the P–H bond is broken and the Ni(II) bonds with a phosphorus atom at the same time. During the formation of IM1, the energy barrier of TS1 is 42.9 kJ mol−1. Then, another P–H bond is broken and the oxygen atom in P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond is hydrogenated by a hydrogen radical. Subsequently, the P–(OH)2 structure is formed as IM3. The calculated highest energy barrier is 132.3 kJ mol−1 in the DRP-I reaction pathway, associated with the formation of IM3. Finally, the P–(OH)2 is attacked by hydrogen and forms P and two H2O by a two-step dehydration reaction.
O bond is hydrogenated by a hydrogen radical. Subsequently, the P–(OH)2 structure is formed as IM3. The calculated highest energy barrier is 132.3 kJ mol−1 in the DRP-I reaction pathway, associated with the formation of IM3. Finally, the P–(OH)2 is attacked by hydrogen and forms P and two H2O by a two-step dehydration reaction.
The direct reduction pathway of hypophosphorous acid via the DRP-II pathway is illustrated in Scheme 1 and Fig. 5. Unlike DRP-I, the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond turns to P–OH via hydrogen intermolecular transfer from a phosphorus atom to an oxygen atom. Specifically, the P–H bond is first broken, followed by the hydrogen transfer from a phosphorus to oxygen atom resulting in the cleavage of a P
O bond turns to P–OH via hydrogen intermolecular transfer from a phosphorus atom to an oxygen atom. Specifically, the P–H bond is first broken, followed by the hydrogen transfer from a phosphorus to oxygen atom resulting in the cleavage of a P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. In this process, the calculated energy barrier is 284.6 kJ mol−1. Afterwards, the bond of P–OH (IM4) breaks and an adsorbed phosphorus atom is formed.
O bond. In this process, the calculated energy barrier is 284.6 kJ mol−1. Afterwards, the bond of P–OH (IM4) breaks and an adsorbed phosphorus atom is formed.
The indirect reduction reaction pathway via the IRP pathway is illustrated in Scheme 1 and Fig. 6. At first, the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond is broken when a hydrogen radical combines with an oxygen atom. In this process, the energy barrier is around 225.9 kJ mol−1. These two double P–OH bonds successively break, releasing two hydroxyl radicals. Eventually, the P–H2 (IM3) breaks and releases two hydrogen radicals. In principle, after overcoming the TS1, the hypophosphorous acid can be rapidly reduced to phosphorus atoms.
O bond is broken when a hydrogen radical combines with an oxygen atom. In this process, the energy barrier is around 225.9 kJ mol−1. These two double P–OH bonds successively break, releasing two hydroxyl radicals. Eventually, the P–H2 (IM3) breaks and releases two hydrogen radicals. In principle, after overcoming the TS1, the hypophosphorous acid can be rapidly reduced to phosphorus atoms.
Obviously, the energy barrier of the DRP-I pathway is much lower than that of DRP-II and IRP. Thus, the DRP-I pathway is considered to be the dominant reduction pathway for the redox of hypophosphorous acid. At the same time, the energy barrier of IRP is close to that of OP (186.5 vs. 225.9 kJ mol−1). As a result, it would be difficult to distinguish these two reactions during electrochemical overpotential measurements.
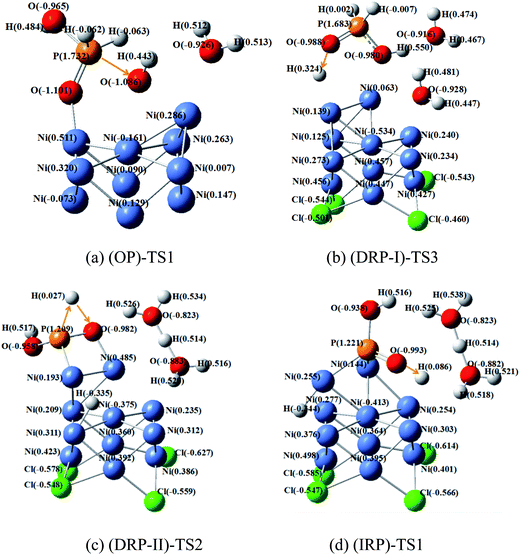
Fig. 7 shows the critical transition states in the highest barrier site for oxidation and reduction pathways. As for OP–TS1, the highest barrier site in oxidation process is OH− combination with hypophosphorous acid. The calculated charges of phosphorus and oxygen atoms are 1.737 and −1.086, respectively. Their charge difference is 2.818, and the barrier of this process is 186.5 kJ mol−1.
The (DRP-I)–TS3, (DRP-II)–TS2 and (IRP)–TS1 are the highest barrier sites for reduction pathways. In these three transition states, hydrogen atoms combine with oxygen atoms to break the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. The charge difference of oxygen in P
O bond. The charge difference of oxygen in P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and hydrogen is 1.312, 1.009, 1079 as shown in Fig. 7b–d, respectively. In addition, the corresponding energy barriers are 132.3, 284.6 and 225.9 kJ mol−1 for (DRP-I)–TS3, (DRP-II)–TS2 and (IRP)–TS1, respectively. Thus, the larger charge difference is favorable for reducing the energy barrier during the hydrogenation of P
O and hydrogen is 1.312, 1.009, 1079 as shown in Fig. 7b–d, respectively. In addition, the corresponding energy barriers are 132.3, 284.6 and 225.9 kJ mol−1 for (DRP-I)–TS3, (DRP-II)–TS2 and (IRP)–TS1, respectively. Thus, the larger charge difference is favorable for reducing the energy barrier during the hydrogenation of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O process.
O process.
3.4 EIS analysis of decomposition of hypophosphorous acid
Fig. 8 reveals the Nyquist impedance spectra of electroless nickel deposition in solution with different pH values. The pH values increase from 3.0 to 9.0 with a step of 2.0. In the pH 3.0 solution, there are two typical time constants, corresponding to two separate capacitive impedance loops in the high and low frequency domains, respectively. At pH 3.0, the sample cannot be deposited by electroless nickel. With a pH increases over pH 5.0, an inductive impedance loop appears in the middle frequency domain and their loops decrease with an increase of pH values in the solution (Fig. 8b). When the pH increases from 5.0 to 9.0, the first capacitive impedance loop gradually increases from 4.42 to 8.60 Ω cm2 in the high frequency domain. | ||
| Fig. 8 Nyquist impedance spectra of EN deposition in solutions with pH values of 3.0, 5.0, 7.0 and 9.0 (a), and a partial spectra magnified image (b). | ||
Fig. 9 shows an equivalent circuit model of Rs(Cdl1Rt1)(ZRt2)(Cdl2Rt3) that was used to simulate the EIS spectra. In the equivalent circuit model, Rs represents the resistance of the electrolyte. There is a typical capacitive loop (CL-H) in the high-frequency domain, which is simulated by Cdl1 and Rt1 in parallel. There is an inductive loop (IL-M) in the medium-frequency domain, consisting of Z and Rt2 in parallel. A capacitive loop (CL-L) was also observed in the low-frequency domain, standing for the Cdl2 and Rt3 in parallel. Fig. 9b exhibits the comparison of experimental data and simulated results, indicative of a good fit. The Nyquist plot was obtained at pH 5.8 in the first hour at the deposition potential. Fig. 10 shows the Nyquist impedance spectra of electroless nickel deposition at different pH values and deposition times. At pH 5.0, there are three time constants observed in the Nyquist plot, including two capacitive loops and one inductance loop. When the pH values increase from 5.0 to 9.0, the diameter of the CL-H loop increases from 5.3 to 25.4 Ω cm2 in the high-frequency domain. These results are in agreement with Touhami's research, suggesting the adsorption of hydroxyl species on the electrode surface.10,11 However, combined with DFT results, the (CL-L) loop corresponds to the Ni–P compound, which closely adsorbs on the Ni surface. At the same time, they are sensitive to hydroxyl, which can combine with a hydrogen radical and lead to the decrease of compound concentration. This result indicates that the doping concentration of phosphorus decreases with an increase of pH. In the meantime, the inductive (IL-M) loop becomes more visible. In addition, the diameter of the CL-L loop increases from 6.5 to 13.2 Ω cm2 in the low-frequency domain. Notably, the resistance of the three electrochemical processes gradually increases as the depositing time increases, due to the decrease of the reactant concentration (e.g., Ni(II) and H2PO2−) and the increase of product concentrations (e.g., H3PO3− and H+) (Table 1).
 | ||
| Fig. 9 An equivalent circuit model (a), and the simulated and measured impedance spectra from a solution with a pH 5.8 bath in the first hour for the EN process (b). | ||
 | ||
| Fig. 10 Nyquist plots of electroless nickel deposition in solutions with pH values of 5.0 (a), 7.0 (b) and 9.0 (c) at different reaction times. | ||
| Pathways | OP | DRP-I | DRP-II | IRP |
|---|---|---|---|---|
| The highest energy barrier/kJ mol−1 | 186.5 | 132.3 | 284.6 | 225.9 |
3.5 Catalytic mechanism of hypophosphorous acid
Combining the results of DFT and EIS, the electroless nickel deposition most likely proceeds through the OP and the DRP-I pathways. They include three typical electrochemical processes, i.e. charge–discharge in electric double layer, adsorption of unstable intermediates on the nickel, and the adsorption of the Ni–P compound intermediates on the nickel. The electrochemical process and relevant species are described in Table 2.Based DFT analysis, the mechanism for the coupling oxidation and reduction reactions from hypophosphorous acid can be illustrated in Scheme 2. During the electroless nickel deposition process, Ni(II) obtains two electrons and turns gradually to Ni(I) and Ni(0). The electrons come from the hydrogen radical, when it combines with a hydroxyl anion. During the oxidation process of hypophosphorous acid, [H3PO2] combines with a hydroxyl anion and releases one hydrogen radical. Thus, a higher hydroxyl anion concentration is favorable for the oxidation process. On the other hand, during the reduction process of hypophosphorous acid, as [H3PO2] releases two hydrogen radicals step-by-step, [HPO2]2+ is able to capture one hydrogen radical and forms [H2PO2]2+. Then the [H2PO2]2+ releases two hydroxyl radicals, resulting in the doping of phosphorus into the nickel films. Importantly, the reduction process needs one hydrogen radical when the hypophosphorous acid switches to a hypophosphate ion. The hydrogen radicals are more stable in a relatively low-pH environment, because they tend to combine with hydroxyl anion. Thus, the reduction process will be facilitated at lower hydroxyl anion concentrations. Overall, the hydroxyl anion is found to be a key species as a switch to control the oxidation and reduction of hypophosphorous acid and a high P-content coating is obtained in a low-pH solution.
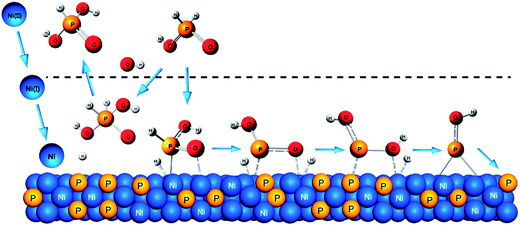 | ||
| Scheme 2 Coupled oxidation and reduction reaction mechanisms of electroless plating nickel from hypophosphorous acid on a nickel surface. | ||
4 Conclusions
This work provide an understanding of how to obtain high-phosphorous non-magnetic electroless nickel coatings, which can be applied as hard disk-drive surface coatings. During the electroless nickel deposition, the doped phosphorus content increases with decreasing pH value. When the pH is lower than 5.0, the coatings start becoming non-magnetic with a phosphorus content over 13.8 wt%. In order to elucidate the phosphorus doping mechanism, the coupled oxidation and reduction reaction pathways of hypophosphorous acid are analyzed by using DFT calculations. The most likely oxidation and reduction reaction pathways are determined to be the OP and DRP-I. Additionally, the electroless nickel process is further illuminated by EIS methods. In particular, there are three electrochemical reaction processes based on the obtained EIS results. The combined EIS and DFT studies can provide new insights into electroless nickel deposition mechanism with controllable phosphorus content. As a result, a mechanism of how the hydroxyl ions inhibit phosphorus doping during the heterogeneous electrocatalytic process is also discussed.Acknowledgements
Dr G. F. Cui gratefully acknowledges the financial support from the National Natural Science Foundation of China (51271205), the “Fundamental Research Funds for the Central Universities” (11lgpy08), the “GuangZhou Pearl Technology the Nova Special Project” (2012J2200058), the “Research and Application of Key Technologies Oriented the Industrial Development” (90035-3283309, 90035-3283321), and the “Plan of Science and Technology Project” of the DaYa Gulf District in Huizhou City (31000-4207387).References
- Z. Qi and W. Lee, Tribol. Int., 2010, 43, 810–814 CAS.
- J. E. A. M. Van den Meerakker, J. Appl. Electrochem., 1981, 11, 395–400 CrossRef CAS.
- T. Homma, I. Komatsu, A. Tamaki, H. Nakai and T. Osaka, Electrochim. Acta, 2001, 47, 47–53 CrossRef CAS.
- H. Nakai, T. Homma, I. Komatsu and T. Osaka, J. Phys. Chem. B, 2001, 105, 1701–1704 CrossRef CAS.
- G. Cui, H. Liu, G. Wu, J. Zhao, S. Song and P. K. Shen, J. Phys. Chem. C, 2008, 112, 4601–4607 CAS.
- A. Brenner, Electrodeposition of Alloys: Principles and Practice. Academic Press, New York, 1963 Search PubMed.
- M. Saitou, Y. Okudaira and W. Oshikawa, J. Electrochem. Soc., 2003, 150, 140–143 CrossRef PubMed.
- R. L. Zeller III and U. Landau, J. Electrochem. Soc., 1991, 138, 1010–1017 CrossRef PubMed.
- T. M. Harris and Q. D. Dang, J. Electrochem. Soc., 1993, 140, 81–83 CrossRef CAS PubMed.
- M. E. Touhami, E. Chassaing and M. Cherkaoui, Electrochim. Acta, 2003, 48, 3651–3658 CrossRef.
- M. E. Touhami, E. Chassaing and M. Cherkaoui, Electrochim. Acta, 1998, 43, 1721–1728 CrossRef CAS.
- T. Homma, A. Tamaki, H. Nakai and T. Osaka, J. Electroanal. Chem., 2003, 559, 131–136 CrossRef CAS.
- T. Homma, H. Nakai, M. Onishi and T. Osaka, J. Phys. Chem. B, 1999, 103, 1774–1778 CrossRef CAS.
- G. Cui, N. Li, D. Li, J. Zheng and Q. Wu, Surf. Coat. Technol., 2006, 200, 6808–6814 CrossRef CAS PubMed.
- G. Cui, N. Li, D. Li and M. Chi, J. Electrochem. Soc., 2005, 152, 669–674 CrossRef PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, J. A. Pople, Gaussian 03 Revision C.02, Gaussian Inc., Pittsburgh PA, 2003 Search PubMed.
- A. D. Becke, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098–3100 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS PubMed.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 299–301 CrossRef CAS PubMed.
- E. Jimenez-Izal, F. Chiatti, M. Corno, A. Rimola and P. Ugliengo, J. Phys. Chem. C, 2012, 116, 14561–14567 CAS.
- M. Jafarian, M. Rashvand avei, M. Khakali, F. Gobal, S. Rayati and M. G. Mahjani, J. Phys. Chem. C, 2012, 116, 18518–18532 CAS.
| This journal is © The Royal Society of Chemistry 2014 |