Delta-mu XANES reveals the electronic structure of the adsorption of propene on gold nanoparticles†
Ana Mijovilovich‡
*
Inorganic Chemistry and Catalysis group, Utrecht University, Universiteitsweg 99, 3584 CG Utrecht, The Netherlands
First published on 19th February 2014
Abstract
Experimental XANES and delta-mu analysis by Nijhuis et al. (2008) have shown that propene adsorption, with pi back-bonding, on gold inhibits the hydrogen oxidation rate in the hydro-epoxidation of propene on gold nanoparticles. In spite of the smallness of the features, delta-mu analysis proved its usefulness in accessing the reaction path. Details of the mechanism are still to be elucidated. The availability of accurate XANES simulation codes enables further analysis. In this work XANES simulations with FEFF9 reproduce the delta-mu spectra for the H oxidized and the propene co-fed Au nanoparticles. The calculations evidence a propene with pi back-bonding to the Au, and bound at defects or steps or in very small Au nanoparticles. The charge on the nanoparticle was analyzed, and the cationic, or even better, the neutrally charged Au nanoparticles reproduce the experiments on the propene co-fed catalyst. Thereby, this work theoretically supports the experimental findings of Nijhuis et al. (2008) and Chen et al. (2013).
A Introduction
In situ XANES spectroscopy studies have suggested the adsorption of propene on supported Au nanoparticles as a critical reaction step in the hydro-epoxidation of propene. More specifically, the co-feeding with propene caused a very strong decrease of the hydrogen oxidation rate. The inhibition of hydrogen oxidation by propene was attributed to the adsorption of propene on the Au nanoparticles. This result was confirmed by delta-mu XANES analysis.1 The adsorption was determined to be pi back-bonding1,2 by comparison with ethene and propane. The adsorption of propene on Au nanoparticles provides additional proof to the finding that Au nanoparticles activate propene to reactively adsorb on titania to give bidentate propoxy species.1 This is a step beyond the already known ability of Au in playing a role in formation of peroxide species.1 However, the detailed mechanism of propene epoxidation is still not yet elucidated and further work is clearly needed.In Nijhuis et al. (2007)1 the catalytic experiments were combined with in situ XANES measurements. Spectra of the hydrocarbon (propene, ethene or propane) co-feeding under reaction conditions (H activation) as well as the spectra in absence of hydrocarbons, were used to show the role of Au nanoparticles in the propene epoxidation reaction mechanism. The conclusions are based on the interpretation of delta-mu XANES, comparing with model cases of ethane or propane co-feeding. In spite of the small effect in the absorption edge, the constant trend found with the development of the reaction over time gives assurance about the experimental conclusions.
The high level of accuracy of XANES simulations at present, allow us to explore some aspects of the mechanism that remain unknown due to the lack of time-resolved experimental studies or inaccessibly reaction steps. The real space multiple scattering theory has been successful in reproducing spectra for Au catalysis.2 The relative edge positions have shown enough accuracy to describe different oxidation states and binding,3–6 in spite of the limitations of using a muffin-tin potential. Herein we report XANES simulations using FEFF93,7 of models of Au nanoparticles in the activated phase, under propene co-feeding and without hydrocarbon treatment. The results provide a theoretical basis to the experimental finding of the binding of propene to the Au nanoparticle as well as is consistent with the pi back-bonding nature of the hydrocarbon bond to the Au. In this work two kinds of Au clusters are analyzed: clusters of the Au(111) surface, the Au/Au(111) to analyze the effect of the edge/defect on a surface, and 5-atom nanoparticles (see Fig. 1). The simple models shown here don't consider the influence of the support directly but only their influence in the charge at the nanoparticle. DFT calculations have shown the support affects the amount of negative charge at the Au catalyst atom, affecting the propene binding: propene binds less strongly to an Au atom adsorbed to an oxygen vacancy in TiO2, due to the additional negative charge.8
B Experimental section
Crystal structures of the Au(111) and the Au/Au(111), and those with propene bound, were taken from Chretien et al.8 For the nanoparticles the 5-atom optimized structures8 with lowest energy of the cationic, neutral and anionic nanoparticles and those with propene bound, were used (see Fig. 1).XANES simulations were performed with FEFF93,7 on clusters of 26 Au atoms for the clusters made from Au(111) and Au/Au(111) (about 10 Å diameter) and on the 5-atom nanoparticles (about 5 Å diameter). The effect of multiple scattering was tested in a cluster of 13 atoms, showing no differences. The code uses a SCF optimization of the potentials, using the muffin-tin prescription with 15% overlap, as the test till 25% didn't show changes. The Hedin–Lundquist exchange correlation potential was used for the excited state and the von Barth for the ground state potential. A 0.5 eV experimental line broadening is added to the XANES core hole line broadening.4–6 The L densities of states for each atom are provided by the calculation.3,9 A test unfreezing the f states of Au did not produce any improvement. Thus, all calculations shown here have frozen f states. Adding a small charge models well the cationic/anionic clusters as well as the H gas treatment.3,10 Computationally, H can also be added as a global charge (as in DFT calculations), moreover if the coordinates aren't known, since its contribution to the multiple scattering is very small due to the low Z. An added charge of 0.025 per Au atom accounted for the H treated phase. The cationic and anionic clusters were simulated adding a small charge of 0.025/−0.025 per Au atom, respectively. The position in energy of the spectral features and their relative intensity was used as estimate of the “goodness” of the simulation.6
Delta-mu spectra for each gas stream were calculated comparing with the spectra of the former step, to check for changes during the treatment:1 for the H activation the comparison is drawn with the sample in He, and for the propene co-feed with the sample during H activation. The sample “in vacuo” was taken as equivalent to the “in He” sample, as the XANES doesn't show differences.1,11
C Results and discussion
C1 Clusters based on Au(111) and Au/Au(111)
Simulations of the Au L3 edge for a cluster of Au(111) of 26 atoms and the propene co-fed Au cluster were rather similar (see Fig. 2A). These simulations do not reproduce well the feature found at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 924 eV in the experiments with propene on hydrogen oxidized Au clusters, as shown in Nijhuis et al.1 (more specifically, it concerns Fig. 4 in that paper), reproduced here in Fig 3. Another simulation with an added Au atom onto the surface of the cluster (Au/Au(111)) reproduced well the feature at 11
924 eV in the experiments with propene on hydrogen oxidized Au clusters, as shown in Nijhuis et al.1 (more specifically, it concerns Fig. 4 in that paper), reproduced here in Fig 3. Another simulation with an added Au atom onto the surface of the cluster (Au/Au(111)) reproduced well the feature at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 924 eV (see Fig. 2B). When propene is bound to the added atom the feature increases. The added atom models a sharp border (steps or defects), which are known to be more reactive than atoms “in the plane”.11
924 eV (see Fig. 2B). When propene is bound to the added atom the feature increases. The added atom models a sharp border (steps or defects), which are known to be more reactive than atoms “in the plane”.11
 | ||
Fig. 2 Simulated XANES of the Au LIII edge for: (A) Au(111), and (B) Au/Au(111) (arrow at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 924 eV). 924 eV). | ||
 | ||
| Fig. 3 Experimental XANES reproduced from Nijhuis et al.1 for: (A) XAFS spectrum of gold/SiO2 catalysts in helium, and (B) zoom of the XANES region of the XAFS spectra in helium, during the hydrogen oxidation, during propene co-feeding in hydrogen oxidation, and after propene removal in hydrogen oxidation (curves overlapping). | ||
The delta-mu of the Au/Au(111) during the H oxidation phase, using the spectra in He for the comparison, shows a negative feature at about 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 920 eV, similarly to Nijhuis et al.1 (see Fig. 4A(a)). The assumption of Nijhuis et al.,1 that the negative peak in delta-mu was due to the shift of the edge to higher energies proved to be true, as illustrated in Fig. 2B. The simulated delta-mu of propene co-fed Au/Au(111), taking the cationic Au/Au(111) (the H oxidized catalyst) for comparison, shows a positive feature at about 11
920 eV, similarly to Nijhuis et al.1 (see Fig. 4A(a)). The assumption of Nijhuis et al.,1 that the negative peak in delta-mu was due to the shift of the edge to higher energies proved to be true, as illustrated in Fig. 2B. The simulated delta-mu of propene co-fed Au/Au(111), taking the cationic Au/Au(111) (the H oxidized catalyst) for comparison, shows a positive feature at about 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 923 eV, a valley at in the range 11
923 eV, a valley at in the range 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930–11
930–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 940 eV and a second peak at higher energies (see Fig. 4A(c)). The simulation using muffin-tin potentials reproduces well the trends in the after-edge region. The feature at 11
940 eV and a second peak at higher energies (see Fig. 4A(c)). The simulation using muffin-tin potentials reproduces well the trends in the after-edge region. The feature at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930 eV is in agreement with the first hour of the gas activation treatment, as shown in Fig. 5 of Nijhuis et al.1 The broad feature at about 11
930 eV is in agreement with the first hour of the gas activation treatment, as shown in Fig. 5 of Nijhuis et al.1 The broad feature at about 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 947 eV in the first stage of the activation is also reproduced in the simulation. At higher energies the match is less accurate, as expected for a non ab initio potentials (muffin-tin). Similarly to the delta-mu of the activated stage of the catalyst, the after-edge range of the simulation reproduces well the initial states of the gas treatments. The FEFF9 simulation reproduces the main feature at the absorption edge.
947 eV in the first stage of the activation is also reproduced in the simulation. At higher energies the match is less accurate, as expected for a non ab initio potentials (muffin-tin). Similarly to the delta-mu of the activated stage of the catalyst, the after-edge range of the simulation reproduces well the initial states of the gas treatments. The FEFF9 simulation reproduces the main feature at the absorption edge.
The effect of the total charge in the catalyst Au/Au(111) was tested using an anionic Au/Au(111) as catalyst (see Fig. 4A(b)). The delta-mu of the propene co-fed anionic Au/Au(111), with the H oxidized anionic Au/Au(111) for comparison, shows a positive feature very similar to that of the propene co-fed cationic Au/Au(111). The propene cofed cationic Au/Au(111), has the delta-mu feature shifted to higher energies than the propene co-fed anionic Au/Au(111), as expected because of the edge shift for the more oxidized sample. The simulation gives an exaggerated enhanced peak at about 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 951 eV instead of the small feature observed in the experiment. However, only the propene co-fed cationic Au/Au(111) show the delta-mu peak at higher energies than the H oxidized, as in the experiment (a 4.3 eV shift). According to these calculations the cationic catalyst gives a closer agreement with the experimental delta-mu peak energy position.
951 eV instead of the small feature observed in the experiment. However, only the propene co-fed cationic Au/Au(111) show the delta-mu peak at higher energies than the H oxidized, as in the experiment (a 4.3 eV shift). According to these calculations the cationic catalyst gives a closer agreement with the experimental delta-mu peak energy position.
In another calculation the delta-mu of the propene co-fed neutral Au/Au(111) cluster, using the neutral cluster for comparison, shows a shift of 1.9 eV in the peak position from the H activated one that is smaller than the cationic cluster (see Fig. 1SA and 1SB at the ESI†). This points out that the neutral cluster reproduces better the peak position found in the experiment of the propene co-fed delta-mu.
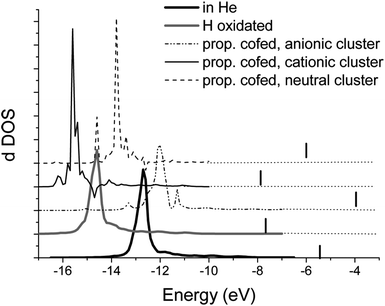
The FEFF9 calculation also provides the LDOS for each differently defined atom. The analogy between the form of XAFS and LDOS means that peaks in the LDOS have a correspondence with a transition between different molecular orbitals.12 According with the “initial state rule” the transition from 2p to the 5d orbital accounts for the transition probability, while the “final state rule” assumes that the LDOS shape is determined in the presence of the core-hole. Van Bokhoven et al.11 have shown that the features at the Au LIII edge XANES difference in O2 or CO are due to changes in the d DOS of the metal. Decreasing the particle size leads to narrower d bands, closer to the Fermi energy. The width of the d DOS increases with an increasing number of total atoms.11 This explains the higher reactivity for Au nanoparticles than bulk Au.
In this work the d DOS of the add atom in Au/Au(111) shows a strong and narrow peak (see Fig. 5). This narrow peak is consistent with a small number of atoms involved, corresponding to the low coordination number of the Au added atom. The Au added atom has the same coordination as the dimer shown by van Bokhoven et al.,11 as well as the same distance of 2.71 Å between the added Au and the bulk. The calculated Ef for all calculated samples (see Table 1 and Fig. 5) appears in a range of about 4 to 8 eV above the (occupied) d DOS peak. Both the propene cofed neutral or the cationic Au/Au(111) shows a d band closer to the Ef than the propene co-fed anionic Au/Au(111) (see Table 1). This means that both the neutral and the cationic Au cluster would be more active than an anionic one. Binding of propene to Au means a transfer from the HOMO in propene to a LUMO in the Au. The easier binding will be that one donating electron charge to the more positive metal, that is, propene on a cationic cluster.8 The added Au atom would facilitate the binding of propene since the metal surface will drag electron density from the added Au atom, making it more positive and then more electrophilic for the propene. Notice that the H oxidized shows the closest d band to Ef, meaning that H absorption and desorption should be easy. Oxidizing the Au cluster with H makes it more positive, then more electrophilic for the propene electrons, in agreement with Bus and van Bokhoven.13 The d DOS amount calculated for propene co-fed, either neutral, cationic or anionic nanoparticle, is reduced with respect to the d DOS of the non hydrocarbon-treated samples (see Table 1). The reduction in the amount of d DOS supports that the binding of the hydrocarbon is pi back-bonding, with a significant charge return to the ligand.11,14
C2 Clusters of 5 atoms
The model used for the Au nanoparticle is scrutinized by comparing with calculations performed on clusters of very few atoms (5 atoms). The lowest energy optimal structures determined by DFT energy minimizations by Chretien et al.8 were used (see Fig. 1B). XANES of the Au anionic, cationic and neutral clusters and their propene co-fed counterparts were simulated with FEFF9.In very small clusters, different cluster charge (cationic, anionic and neutral) causes a rearrangement of the structure. The calculated delta-mu of the cationic H oxidized, taking the “in He” for comparison, and the propene co-fed cationic cluster, taking the H oxidized for comparison, both reproduce well the experimental results (see Fig. 4B). While for Au nanoparticles of small size, a rearrangement of the structure occurs for different charges (cationic, anionic or neutral cluster), for a bigger Au particle the small change of charge during the H activation would be shared in a metal-like manner, without a significant rearrangement of the particle structure. In this work the delta-mu of the cationic propene co-fed and the H oxidized seems to not be affected by the different structures of the 5-atom nanoparticles and the Au/Au(111) clusters.
The case of the propene co-fed anionic nanoparticles, taking for comparison the anionic nanoparticle, shows a tiny shift to lower energies of the main peak. While the total charge sign resulted in a significant shift of the peak for the Au/Au(111) delta-mu, it seems unimportant for the 5-atom nanoparticles.
D Conclusions
From the IR experiments of Chen et al.15 it is argued that the H oxidized Au cluster is neutrally charged and the low coordinated Au becomes negatively charged in presence of propene. In this work a calculation for the neutral 5-atom nanoparticles propene co-fed delta-mu shows a smaller change in the peak position from the H oxidized one (see Fig. 1SC at the ESI†). This is similar to the result on the neutral and charged Au/Au(111) clusters discussed above.The results show that either the neutral cluster after the H oxidation, as in Chen et al.,15 or the cationic cluster, give a shift in the delta-mu as observed by Nijhuis et al.1 For the Au/Au(111) clusters, also it is the neutral cluster after the H oxidation peak energy shift, of about 1.9 eV, which shows a good agreement with the experimental shift1 of 2.2 eV, while the H oxidized cationic cluster shift of 4.3 eV doubles the experimental value.
Summarizing, the real space multiple scattering XANES simulations with FEFF9 reproduce well the experimental delta-mu of Nijhuis et al.1 The features found at the absorption edge of the H oxidized and the propene co-fed on Au can only be reproduced when the gases are adsorbed on very small nanoparticles or defects/edges of a bigger Au cluster, but not when adsorbed on bulk Au metal. The feature in the XANES at about 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 924 eV does not appear in the H oxidized cluster, but only when propene is co-fed. Thus, the XANES calculations explain the experimental findings that the feature is due to the binding of propene.1 The experimental shift in the main peak of the delta-mu of the propene co-fed, with respect to that of the H oxidized one, is better reproduced by the neutral H cluster than the charged ones. The finding is similar for clusters made from Au/Au(111) or the 5-atom nanoparticles. Furthermore, the calculated d DOS for the metal decreases for the propene co-fed Au cluster, indicating there is pi back-bonding.
924 eV does not appear in the H oxidized cluster, but only when propene is co-fed. Thus, the XANES calculations explain the experimental findings that the feature is due to the binding of propene.1 The experimental shift in the main peak of the delta-mu of the propene co-fed, with respect to that of the H oxidized one, is better reproduced by the neutral H cluster than the charged ones. The finding is similar for clusters made from Au/Au(111) or the 5-atom nanoparticles. Furthermore, the calculated d DOS for the metal decreases for the propene co-fed Au cluster, indicating there is pi back-bonding.
Abbreviations
| XANES | X-ray absorption near edge structure; |
| DFT | Density functional theory; |
| LDOS | L densities of states, or DOS. |
Acknowledgements
Dr Elena Sacaliuc-Parvulescu and Prof. Dr Bert Weckhuysen are acknowledged for useful discussions. We thank Prof. Horia Metiu and Dr Steeve Chretien from the Dept of Chemistry and Biochemistry at U.C. Santa Barbara (USA) for kindly releasing the crystal structures. Dr Cinthia Ramos at U.A. Fisica, CNEA (Argentina) and Prof. Dr T.A. Nijhuis from Chemical Reactor Engineering at Eindhoven University of Technology (The Netherlands) are acknowledged for their kind support of this research work.Notes and references
- T. A. Nijhuis, E. Sacaliuc, A. M. Beale, A. M. J. van der Eerden, J. C. Schouten and B. M. Weckhuysen, J. Catal., 2008, 258, 256–264 CrossRef CAS PubMed.
- E. Bus, D. E. Ramaker and J. A. Van Bokhoven, J. Am. Chem. Soc., 2007, 129, 8094–8102 CrossRef CAS PubMed.
- A. L. Ankudinov, B. Ravel, J. J. Rehr and S. D. Conradson, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 7565–7576 CrossRef CAS.
- A. Mijovilovich and W. Meyer-Klaucke, J. Synchrotron Radiat., 2003, 10, 64–68 CrossRef CAS PubMed.
- A. Mijovilovich, Chem. Biodiversity, 2008, 5, 2131–2139 CAS.
- A. Mijovilovich, H. Hayashi, N. Kawamura, H. Osawa, P. C. A. Bruijnincx, R. J. M. Klein Gebbink, F. M. F. de Groot and B. M. Weckhuysen, Eur. J. Inorg. Chem., 2012, 2012, 1589–1597 CrossRef CAS.
- J. J. Rehr, J. J. Kas, F. D. Vila, M. P. Prange and K. Jorissen, Phys. Chem. Chem. Phys., 2010, 12, 5503–5513 RSC.
- S. Chretien, M. S. Gordon and H. Metiu, J. Chem. Phys., 2004, 121, 3756–3766 CrossRef CAS PubMed.
- A. Mijovilovich, S. Hamman, F. Thomas, F. M. F. de Groot and B. M. Weckhuysen, Phys. Chem. Chem. Phys., 2011, 13, 5600–5604 RSC.
- A. L. Ankudinov, J. J. Rehr, J. Low and S. R. Bare, Phys. Rev. Lett., 2001, 86, 1642–1645 CrossRef CAS.
- J. A. Van Bokhoven and J. T. Miller, J. Phys. Chem. C, 2007, 111, 9245–9249 CAS.
- J. J. Rehr and A. L. Ankudinov, Coord. Chem. Rev., 2005, 249, 131–140 CrossRef CAS PubMed.
- E. Bus and J. A. Van Bokhoven, Phys. Chem. Chem. Phys., 2007, 9, 2894–2902 RSC.
- P. Frank, P. Ghosh, K. O. Hodgson and H. Taube, Inorg. Chem., 2002, 41, 3269–3279 CrossRef CAS PubMed.
- J. Chen, E. A. Pidko, V. Ordomsky, T. Verhoeven, E. J. M. Hensen, J. C. Schouten and T. A. Nijhuis, Catal. Sci. Technol., 2013, 3, 3042–3055 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Figure of the delta-mu of the neutral clusters. See DOI: 10.1039/c3ra47209d |
| ‡ Present address: Visiting scientist at Instituto de Investigaciones Físicas de Mar del Plata (CONICET-UNMdP)Funes 3350 - (7600) Mar del Plata, Argentina. E-mail: E-mail: ana_mijo@yahoo.com |
| This journal is © The Royal Society of Chemistry 2014 |