A one-pot sol–gel process to prepare a superhydrophobic and environment-resistant thin film from ORMOSIL nanoparticles†
Xinxiang Zhang*a,
Feng Zhenga,
Longqiang Yeb,
Pan Xiongb,
Lianghong Yanc,
Wenbin Yanga and
Bo Jiangb
aCollege of Materials Engineering, Fujian Agriculture and Forestry University, Fuzhou, 350002, China. E-mail: xxzhang0106@163.com; Fax: +86-951-83715175; Tel: +86-951-83715175
bKey Laboratory of Green Chemistry & Technology, College of Chemistry, Sichuan University, Chengdu, 610064, China
cResearch Center of Laser Fusion, China Academy of Engineering Physical, Mianyang, 621900, China
First published on 28th January 2014
A one-pot sol–gel process was used to prepare an ORMOSIL thin film which consisted of ORMOSIL nanoparticles. This thin film featured superhydrophobicity, excellent environmental resistance and transmittance making it a competitive candidate as antireflective coating in high power laser systems.Sol–gel silica antireflective (AR) coatings have been widely used in optical devices and energy-related applications to reduce light reflection.1–4 The refractive index of sol–gel silica AR coating is equal to the square root of that of many common optical glasses, giving the coated glasses nearly 100% transmission.5 However, sol–gel silica AR coatings are hydrophilic and possess high specific surface areas, and therefore tend to adsorb contamination, such as water or polar organic molecules, from the environment.6 The contaminants adsorbed into the pores of the AR coatings will degrade the optical property, which was known as poor environmental resistance of the hydrophilic silica AR coating. For several decades, great effort has been devoted to prepare organically modified silicates (ORMOSIL) thin film with improved hydrophobic property and hence environmental resistance by hydrophobic modification.7–14 The optimum goal of hydrophobic modification is to realize superhydrophobicity. It is well known that it is the combination of surface roughness and low-surface-energy modification that leads to superhydrophobicity. Our researches are committed to provide high quality sol–gel silica AR coating for high power laser systems. The transmittance of AR coating coated substrates in high power laser systems should be higher than 99.5%. The idea is accepted commonly that superhydrophobic surface requires hierarchical structures to improve surface roughness.15 This indicates that it is very difficult to prepare superhydrophobic silica AR coatings for the laser systems because the superhydrophobicity and excellent transmittance are well known to be competitive due to the extensive light scattering effect on rough surface.16
Recently, we reported a simple template-free sol–gel route for preparation of superhydrophobic ORMOSIL thin film with excellent transmittance of higher than 99.6% by surface modification of silica nanonanoparticles using hexamethylisilazane (HMDS).10 Although the environmental resistance of this ORMOSIL thin film was greatly improved, it would still tend to adsorb contaminants from environment, indicating that superhydrphobicity will not necessarily afford the thin film excellent environmental resistance. Hydrophobic methyl-substituted silane precursors such as methyltriethoxysilane (MTES) or dimethyldiethoxysilane (DDS) were widely used to partially substituted TEOS to prepare environment-resistant ORMOSIL thin film.7,8
In this work, we prepare a superhydrophobic ORMOSIL thin film with excellent environmental resistance by a two-step one-pot sol–gel process. In the first step, ORMOSIL thin film was prepared using tetraethylorthosilicate (TEOS) and DDS as co-precursor by a typical Stöber sol–gel method. In the second step, the hydrophobicity of above ORMOSIL thin film is further increased to superhydrophobicity by the surface modification of ORMOSIL nanonanoparticles with HMDS. TEOS, DDS, EtOH, H2O and NH3 H2O was added in a seal glass container and then immediately stirred for 2 hours at 30 °C. The molar ratio of (TEOS + DDS)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH
EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3 was 1
NH3 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.25
3.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37.6
37.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.17. This produced sols of 3% equivalent SiO2. After aging for 2 weeks, the sols were diluted to 1.5% to obtain silica sols with a suitable viscosity for coating a uniform thin film by the dip-coating process. The silica sols were labeled as S0, S0.1, S0.2, S0.4 and S0.6 and resultant thin films were referred as F0, F0.1, F0.2, F0.4 and F0.6 as the molar ratio of DDS to TEOS is 0, 0.1, 0.2, 0.4 and 0.6, respectively. HMDS was added into S0, S0.1, S0.2, S0.4 and S0.6, and aged at 25 °C for more than 7 days. The weight ratio of HMDS to silica is 100%. The HMDS-modified sols were labeled as SM0, SM0.1, SM0.2, SM0.4 and SM0.6 and resultant thin films as FM0, FM0.1, FM0.2, FM0.4 and FM0.6.
0.17. This produced sols of 3% equivalent SiO2. After aging for 2 weeks, the sols were diluted to 1.5% to obtain silica sols with a suitable viscosity for coating a uniform thin film by the dip-coating process. The silica sols were labeled as S0, S0.1, S0.2, S0.4 and S0.6 and resultant thin films were referred as F0, F0.1, F0.2, F0.4 and F0.6 as the molar ratio of DDS to TEOS is 0, 0.1, 0.2, 0.4 and 0.6, respectively. HMDS was added into S0, S0.1, S0.2, S0.4 and S0.6, and aged at 25 °C for more than 7 days. The weight ratio of HMDS to silica is 100%. The HMDS-modified sols were labeled as SM0, SM0.1, SM0.2, SM0.4 and SM0.6 and resultant thin films as FM0, FM0.1, FM0.2, FM0.4 and FM0.6.
Fig. 1 illustrates the growth process of pure silica nanoparticles by hydrolysis and condensation of TEOS, ORMSIL nanoparticles by hydrolysis and co-condensation of DDS and TEOS and nanoparticles further modified by HMDS. As shown in process (a) of Fig. 1, silica nanoparticles are formed by hydrolysis and condensation of TEOS mixing with water and ammonia in ethanol solution. TEOS is first hydrolyzed, and then the free hydroxyl groups dehydrate to form larger molecules. The condensation reaction continues in three dimensions, and eventually results in silica nanoparticles.17 The surface of silica nanoparticles from TEOS is covered by hydroxyl groups. These hydroxyl groups can be replaced by –OSi(CH3)3 groups of HMDS (as shown in process (c) of Fig. 1). The formation of ORMOSIL nanoparticles from TEOS and DDS is similar to that of silica nanoparticles from TEOS. However, the surface of ORMOSIL nanoparticles is covered with not only hydroxyl groups but also methyl groups because TEOS is partially replaced by DDS (as shown in process (b) of Fig. 1).
 | ||
| Fig. 1 Schematic representation of growth process of pure silica nanoparticles from TEOS (a), ORMOSIL nanoparticles from TEOS and DDS (b), and nanoparticles further modified by HMDS (c and d). | ||
Fourier transform infrared (FTIR) spectra of silica powers were obtained with a Bruker Tensor 27 using KBr method in transmission mode and shown in Fig. 2. All spectra showed a very strong absorption band at 1084 cm−1 and a medium intensity band at 806 cm−1 those are typical of silica from the sol–gel process. These two bands are attributed to the Si–O–Si bond corresponding to bending and stretching vibrations, respectively.18 By the contrast of the FTIR spectra of S0, S0.2 and S0.6, it can be found that the additional absorption bands at 1265 cm−1 and 850 cm−1 corresponding to the Si–CH3 stretching19 and bending20 vibrations appear for DDS-containing silica samples (S0.2 and S0.6). With increase of DDS content, the Si–CH3 absorption band strengthens. In addition, absorption bands corresponding to C–H stretching modes at 2963 cm−1 and 2905 cm−1 were also strengthened significantly. In conclusion, FTIR spectra confirmed that lots of hydrophobic methyl groups were introduced into silica thin film by the hydrolysis and co-condensation of DDS and TEOS.
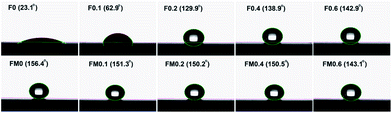
The change of the water contact angle values of the silica thin films with DDS content was shown in Fig. 3. As the molar ratio of DDS to TEOS increases from 0 to 0.6, the water contact angles of silica thin films increased from 23.1° to 142.9°. This is in accordance with the previous representation of particle growth process shown in Fig. 1. F0 is hydrophilic because its surface is totally surrounded by hydrophilic hydroxyl groups. By replacing TEOS with DDS gradually, more and more hydrophobic methyl groups are introduced into framework and onto surface of silica nanoparticles. Silica thin films with more hydrophobic methyl groups have lower surface energy, and hence higher water contact angle. It can be concluded that DDS is a good hydrophobic modifier for silica thin film prepared from TEOS. The water contact angle of silica thin film increases obviously from 23.1° to 129.9° after only replacing 16.7% TEOS with DDS (the molar ratio of DDS to TEOS is 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The water contact angle of F0.6 is close to 150°, which reveals that F0.6 represents a nearly superhydrophobic surface.15
1). The water contact angle of F0.6 is close to 150°, which reveals that F0.6 represents a nearly superhydrophobic surface.15
The aim of our work was to prepare environment-resistant silica thin film for high power laser system. In our previous work, superhydrophobic silica thin film was prepared by surface modification of silica nanoparticles with HMDS.10 As shown in process (c) of Fig. 1, the surface of HMDS-modified silica nanoparticles is totally covered by –Si(CH3)3. It was demonstrated that the environmental resistance of this thin film was significantly improved. However, after a long-term usage, the transmittance of this thin film would still decrease slightly. The decrease in transmittance is attributed to the adsorption of contaminants from environment by vapor adsorption. It was generally considered that the environmental resistance is related to the hydrophobicity which is revealed by water contact angle. However, our previous work revealed that superhydrophobicity would not necessarily afford silica thin film excellent environmental resistance. This is because the silica thin film tends to adsorb contaminants from environment by vapor adsorption. Superhydrophobicity is the surface property. Thin film with superhydrphobic property can absolutely prevent liquid water from penetrating into silica thin film, but not vapor contaminants. To realize excellent environmental resistance, the key is to introduce amounts of hydrophobic groups into silica framework but not to only introduce hydrophobic groups onto surface of silica nanoparticles. As shown in Fig. 2, although FM0 is superhydrophobic, SM0 showed weak absorption bands at 1265 cm−1 and 850 cm−1 corresponding to the Si–CH3, indicating that there is few hydrophobic methyl groups were introduced into silica nanoparticles. Compared with S0, SM0.2 and SM0.6 showed very strong absorption bands of methyl groups. This is the reason that researches were inclined to prepare environment-resistant silica thin film by co-condensation of TEOS with methyl-substituted silane precursors.7,8,14 Xu's research7 revealed that ORMOSIL thin films by co-condensation of TEOS with MTES possess excellent environment resistance, and the transmittance of these thin films decreased slightly from 100% to 99.8% after 220 days' aging. As shown in Fig. 3, the surface modification of S0.6 silica nanoparticles did not increase the water contact angle, indicating that there are almost no hydroxyl groups on surface of S0.6 silica nanoparticles. In addition, FTIR spectrum of S0.6 showed that lots of hydrophobic methyl groups were introduced into ORMOSIL particles. Therefore, F0.6 might be the most competitive candidate as environment-resistant ORMOSIL thin film.
The transmittance is the most important property for silica thin film used in high power laser system. Silica thin film prepared by sol–gel process consists of a layer of silica nanoparticles, randomly stacked on the optics' surface. The particle size of silica nanoparticles is very important because large silica nanoparticles will result in light scatter. The particle size of silica thin film in National Ignition Fusion (NIF) is about 20 nm to guarantee thin film with excellent transmittance (higher than 99.5%). Particle sizes of S0, S0.1, S0.2, S0.4 and S0.6 are characterized by DLS and showed in Fig. 4. The particle size of S0.6 is about 44 nm, which we believe is too big to use in high power laser system. Therefore, we combined the co-condensation of TEOS and DDS with further surface modification of silica nanoparticles by HMDS to prepare a superhydrophobic ORMOSIL thin film with excellent environment resistance and transmittance. The co-condensation of TEOS and DDS introduces lots of hydrophobic methyl groups into framework of silica nanoparticles, and the surface modification replaces hydroxyl groups on silica nanoparticles with –Si(CH3)3groups. The water contact angle of FM0.4 is higher than 150°, and the nanoparticle size of S0.4 is about 24 nm. Xu et al.7 had prepared ORMOSL AR coatings with excellent environmental resistance by co-condensation of TEOS and MTES or DDS. However, only ORMOSIL thin film from TEOS and MTES was used as environment-resistant AR coatings. ORMOSIL thin film from TEOS and DDS (i.e. FM0.4 in this work) possesses poor transmittance due to its ultra-low refractive index (see ESI†). In order to use this ORMOSIL thin film in high power laser system, a second layer between the glass substrate and ORMOSIL AR coating to realize 100% transmittance. The detail discussions were shown in ESI.† Finally, the environmental resistance of ORMOSIL thin film was characterized according to Xu's method.7 Generally speaking, DDS is a more effective hydrophobic modifier than MTES because it has one more methyl groups. The excellent environmental resistance of FM0.4 is in line with what we expected. After a long-term aging, the transmittance of ORMOSIL AR coating is still above 99.5%.
In conclusion, we report here a simple one-pot sol–gel method to prepare ORMOSIL thin film by using TEOS and DDS as co-precursor and HDMS as surface modifier. This ORMOSIL thin film realized superhydrophobicity by introducing amounts of hydrophobic methyl groups by co-condensation of TEOS and DDS and further replacing hydroxyl groups on surface of ORMOSIL nanoparticles with hydrophobic –Si(CH3)3 groups. The particle size of ORMOSIL nanoparticles was about 24 nm which will not result in light scatter when the ORMOSIL thin film used as antireflective (AR) coating. This superhydrophobic ORMOSIL thin film with excellent environmental resistance and transmittance can find significant application in high-powered fusion laser system.
Acknowledgements
The authors gratefully acknowledge the support from Natural Science Foundation of China (31770535).Notes and references
- H. K. Raut, V. A. Ganesh, A. S. Nair and S. Ramakrishna, Energy Environ. Sci., 2011, 4, 3779–3804 CAS
.
- A. Yildirim, H. Budunoglu, M. Yaman, M. Guler and M. Bavindir, J. Mater. Chem., 2011, 21, 14830–14837 RSC
.
- G. Zhou, J. H. He, L. J. Gao, T. T. Ren and T. Li, RSC Adv., 2013, 3, 21789–21796 RSC
.
- A. L. Pénard, T. Gacoin and J. P. Boilot, Acc. Chem. Res., 2007, 40, 895–902 CrossRef PubMed
.
- P. K. Whitman, S. C. Frieders, J. Fair, I. M. Thomas, R. Aboud, C. B. Thorsness and A. K. Burnham, ICF Quarterly Report, 1999, 9, 163–176 Search PubMed
.
- I. M. Thomas, A. K. Burnham, J. R. Ertel and S. C. Frieders, Proc. SPIE, 1999, 3492, 220 CrossRef CAS PubMed
.
- Y. Xu, L. Zhang, D. Wu, Y. H. Sun, Z. X. Huang, X. D. Jiang, X. F. Wei, Z. H. Li, B. Z. Dong and Z. H. Wu, J. Opt. Soc. Am. B, 2005, 22, 905–912 CrossRef CAS
.
- Y. Zhang, D. Wu, Y. Sun and S. Peng, J. Sol-Gel Sci. Technol., 2005, 33, 19–24 CrossRef
.
- M. Manca, A. Cannavale, L. Marco De, A. S. Aric, R. Cingolani and G. Gigli, Langmuir, 2009, 25, 6357–6362 CrossRef CAS PubMed
.
- X. X. Zhang, S. Cai, D. You, L. H. Yan, H. B. Lv, X. D. Yuan and B. Jiang, Adv. Funct. Mater., 2013, 23, 4361–4365 CrossRef CAS
.
- X. X. Zhang, C. R. Cao, B. Xiao, L. H. Yan, Q. H. Zhang and B. Jiang, J. Sol-Gel Sci. Technol., 2010, 53, 79–84 CrossRef CAS PubMed
.
- X. Zhang, B. Xia, H. Ye, Y. Zhang, B. Xiao, L. Yan, H. Lv and B. Jiang, J. Mater. Chem., 2012, 22, 13132–13140 RSC
.
- B. Xiao, B. Xia, H. Lv, X. Zhang and B. Jiang, J. Sol-Gel Sci. Technol., 2012, 64, 276–281 CrossRef CAS
.
- X. Wang and J. Shen, J. Sol-Gel Sci. Technol., 2012, 61, 206–212 CrossRef CAS
.
- X. Zhang, F. Shi, J. Niu, Y. G. Jiang and Z. Q. Wang, J. Mater. Chem., 2008, 18, 621–633 RSC
.
- L. G. Xu, L. J. Gao and J. H. He, RSC Adv., 2012, 2, 12764–12769 RSC
.
- X. Zhang, H. Ye, B. Xiao, L. Yan, H. Lv and B. Jiang, J. Phys. Chem. C, 2010, 114, 19979–19983 CAS
.
- M. J. Mosquera, D. M. Santos, A. Montes and L. Valdez-Castro, Langmuir, 2008, 24, 2772–2778 CrossRef CAS PubMed
.
- N. Wright and M. J. Honter, J. Am. Chem. Soc., 1947, 69, 803 CrossRef CAS
.
- N. Hering, K. Schriber, R. Riedel, O. Lichtenberger and J. Woltersodorf, Appl. Organomet. Chem., 2001, 15, 879 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: The transmittance of FM0.4. See DOI: 10.1039/c3ra47185c |
| This journal is © The Royal Society of Chemistry 2014 |