Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile preparation of indoxyl- and nitrophenyl glycosides of lactosamine and isolactosamine†
Stephan Böttcher and
Joachim Thiem*
Department of Chemistry, Faculty of Science, University of Hamburg, Martin-Luther-King-Platz 6, 20146 Hamburg, Germany. E-mail: thiem@chemie.uni-hamburg.de
First published on 24th January 2014
Abstract
The synthesis of the novel indoxyl glycosides of N-acetyl-lactosamine (X-LacNAc) and N-acetyl-isolactosamine (X-LNB) is reported employing glycosyl chlorides in a facile phase transfer glycosylation, followed by mild decarboxylation and finally deacetylation. Correspondingly the ortho-nitrophenol and para-nitrophenol glycosides of LacNAc and LNB could be obtained.
Introduction
N-Acetyl-lactosamine (LacNAc, Galβ1-4GlcNAc) and N-acetyl-isolactosamine (LNB, Galβ1-3GlcNAc) are integral parts of biologically very important glycostructures. Both represent essential subunits of complex milk oligosaccharides1,2 as well as of antigens3–5 and glycoconjugates.6,7Access to samples or intermediates of complex glycostructures is still rather difficult. The chemical synthesis of complex saccharide structures is loaded with barriers, regarding protecting group chemistry as well as control of stereo- and regio-chemistry. Enzymatic syntheses need fewer steps, are often highly stereo- and regio-selective and are therefore superior if available. Thus, these approaches complement the array of methods, however, often enzymes are not available or difficult to isolate, purify or handle.
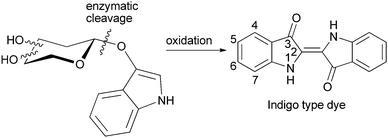
A convenient method for screening of glycosidases as well as transglycosidases is to use indoxyl glycosides (Fig. 1). Indoxyl is released by enzymatic cleavage of the glycosidic linkage and then rapidly oxidized, e.g. by atmospheric oxygen, to an indigo type dye. Common substrates are halogenated, as the substitution pattern determines colour and physical properties of the resulting indigo dye. The most common pattern of indoxyls are 5-bromo-4-chloro, 5-bromo and 5-bromo-6-chloro derivatives.8 Nitrophenol glycosides in turn are widely utilised compounds for activity measurements and are employed as convenient donor substrates in enzymatic syntheses.9–12
Here we wish to report convenient syntheses of novel indoxyl glycosides of N-acetyl-lactosamine (X-LacNAc) and N-acetyl-isolactosamine (X-LNB), as well as the synthesis of para-nitrophenyl and ortho-nitrophenyl glycosides of both disaccharides.
The peracetates of LacNAc and LNB were converted into the corresponding glycosyl chlorides. The crude products could be used after washing directly without further workup for phase transfer glycosylation with the respective nitrophenol acceptor. The oNP and pNP glycosides were obtained after Zemplén deacetylation13 in good overall yields. Preparation of the indoxyl glycosides followed our recently developed approach. In a phase transfer glycosylation indoxylic acid allyl esters were used as acceptors, followed by selective mild silver mediated decarboxylation and finally Zemplén deacetylation.14,15
Results and discussion
The synthetic route for the preparation of LNB-oNP/pNP (5a/b) and LacNAc-oNP/pNP (5c/d) is summarized in Scheme 1.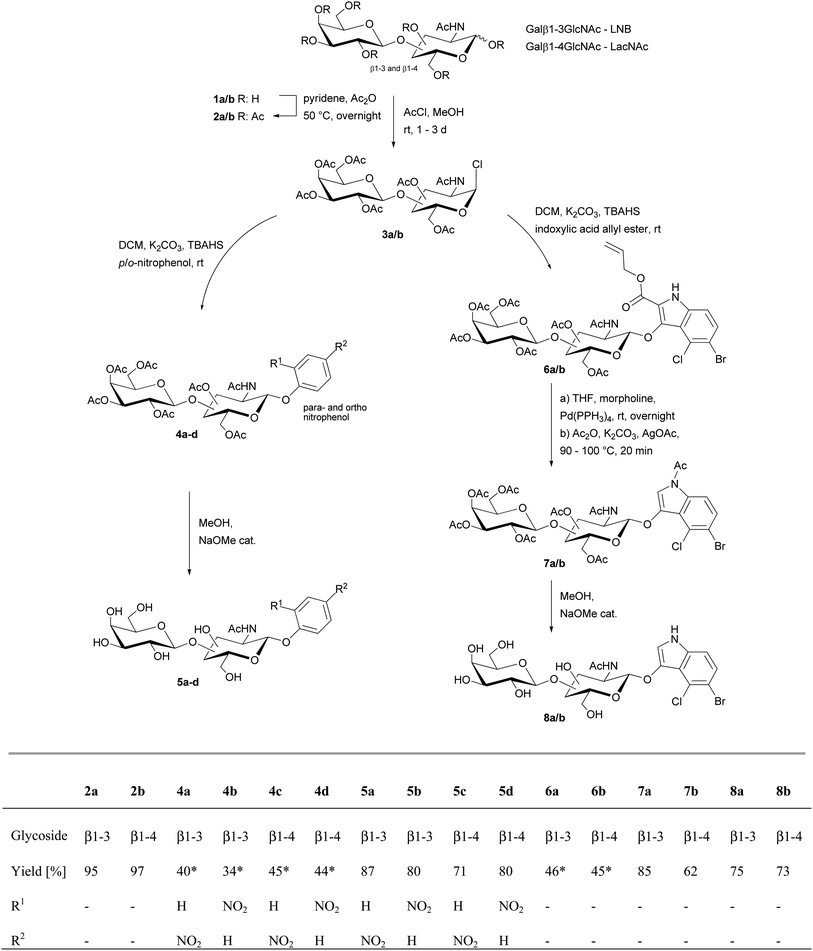 | ||
| Scheme 1 Synthesis of p/o-nitrophenol glycosides of isolactosamine (5a/b) and lactosamine (5c/d) as well as indoxyl glycosides of isolactosamine (8a) and lactosamine (8b). *Yield via two steps. | ||
The peracetates 2a/b were treated with acetyl chloride and methanol under argon atmosphere to give the glycosyl chlorides 3a/b. These donors were dried and washed to remove acetyl chloride, and were then subjected to phase transfer glycosylation without further workup to yield peracetylated LNB-pNP (4a, 40%), LNB-oNP (4b, 34%), LacNAc-pNP (4c, 45%), LNB-oNP (4d, 44%) via two steps. Finally, the acetyl protecting groups were removed by Zemplén deacetylation.13 In case of 5d the reaction mixture needed to be kept at 45 °C overnight to achieve complete deacetylation. All glycosides crystallised during deacetylation and were washed after filtration with a small amount of cold methanol to give very pure 5a–d in good yields.
The synthesis of the indoxyl glycosides 8a/b was again based on the conversion of the respective peracetate 2a or 2b to the crude glycosyl chlorides 3a/b. Phase transfer glycosylation under common phase transfer conditions gave 6a (46%) and 6b (45%). Then selective allyl ester deprotection with Pd(PPh3)4 and morpholine in THF16 followed by mild silver mediated decarboxylation14,15 gave the acetylated glycosides 7a (85%) ad 7b (62%). Finally Zemplén deacetylation13 was used to give the novel indoxyl glycosides X-LNB in 75% and X-LacNAc in 73% yield.
Conclusions
Within this work we could elaborate facile syntheses of novel indoxyl glycosides X-LAcNAc and X-LNB as well as the corresponding ortho- and para-nitrophenyl glycosides of LacNAc and LNB. Both reaction pathways could be carried out in good yields, employing facile phase transfer glycosylation of the crude glycosyl chloride donors, straight forward workup and crystallization.Experimental section
General remarks
All reagents were purchased from commercial sources and used as received. TLC was performed on Merck silica gel 60 F254 plates. Compounds were detected by UV and/or by treatment with EtOH/H2SO4 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and subsequent heating. Column chromatography was performed with Merck/Fluka silica gel 60 (230–400 mesh). Solvents for column chromatography were distilled prior to use. 1H and 13C NMR spectra were recorded with Bruker AMX-400 or Bruker AV-400 spectrometers (400 MHz for 1H, 101 MHz for 13C) and calibrated using the solvent residual peak. In CDCl3 TMS was used for calibration. Melting points were measured with an Büchi melting point M-565. Optical rotations were obtained using a Krüss Optronic P8000 polarimeter (589 nm). HRMS (ESI) were recorded with a Thermo Finnigan MAT 95XL mass spectrometer.
1) and subsequent heating. Column chromatography was performed with Merck/Fluka silica gel 60 (230–400 mesh). Solvents for column chromatography were distilled prior to use. 1H and 13C NMR spectra were recorded with Bruker AMX-400 or Bruker AV-400 spectrometers (400 MHz for 1H, 101 MHz for 13C) and calibrated using the solvent residual peak. In CDCl3 TMS was used for calibration. Melting points were measured with an Büchi melting point M-565. Optical rotations were obtained using a Krüss Optronic P8000 polarimeter (589 nm). HRMS (ESI) were recorded with a Thermo Finnigan MAT 95XL mass spectrometer.
General procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) indicated no further reaction. The solvent was removed under reduced pressure and the crude product was dissolved in DCM, concentrated again and washed with of diisopropyl ether (25 mL). The product was used directly according to general procedure 2 without further workup.
7) indicated no further reaction. The solvent was removed under reduced pressure and the crude product was dissolved in DCM, concentrated again and washed with of diisopropyl ether (25 mL). The product was used directly according to general procedure 2 without further workup.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, RF: 0.45; starting material 0.40). (2) DCM (60 mL), aqueous K2CO3 solution (60 mL, 1 M), TBAHS (1.50 g, 4.42 mmol), p-nitrophenol (620 mg, 4.46 mmol). Yield 40% (1.35 g, 1.78 mmol); colorless solid; mp 129 °C; [α]25D -27.8 (c 0.50 in CHCl3); RF 0.69 (heptane/acetone 3
7, RF: 0.45; starting material 0.40). (2) DCM (60 mL), aqueous K2CO3 solution (60 mL, 1 M), TBAHS (1.50 g, 4.42 mmol), p-nitrophenol (620 mg, 4.46 mmol). Yield 40% (1.35 g, 1.78 mmol); colorless solid; mp 129 °C; [α]25D -27.8 (c 0.50 in CHCl3); RF 0.69 (heptane/acetone 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7); 1H-NMR (400 MHz, CDCl3) δ 8.21–8.16 (2H, m, Harom), 7.10–7.04 (2H, m, Harom), 5.93 (1H, d, J = 7.5 Hz, NH), 5.71 (1H, d, J1,2 = 7.0 Hz, H-1), 5.37–5.35 (1H, m, H-4′), 5.12 (1H, dd, J1′,2′ = 7.8 Hz, J2′,3′ = 10.4 Hz, H-2′), 5.06 (1H, dd∼vt, H-4), 4.99 (1H, dd, J2′,3′ = 10.4 Hz, J3′,4′ = 3.4 Hz, H-3′), 4.64 (1H, d, J1′,2′ = 7.8 Hz, H-1′), 4.57 (1H, dd∼vt, H-3), 4.24 (1H, dd, J5,6a = 6.1 Hz, J6a/b = 12.3 Hz, H-6a), 4.19–4.07 (3H, m, H-6b, H-6′a/b), 3.98–3.88 (2H, m, H-5, H-5′), 3.67 (1H, ddd∼vdd, H-2), 2.16, 2.09, 20.8, 2.07, 2.00, 1.98 (each 3H, s, C(O)CH3), 2.02 (3H, s, NHC(O)CH3); 13C-NMR (100 MHz, CDCl3) δ 170.7, 170.4, 170.1, 170.1, 169.1, 169.1 (C(O)CH3), 161.4 (NHC(O)CH3), 143.0 (Cquaternary), 125.7, 116.5 (CHarom), 100.7 (C-1′), 96.3 (C-1), 76.0 (C-3), 72.4 (C-5), 70.9, 70.8 (C-3′, C-5′), 69.4 (C-2′), 68.5 (C-4), 66.8 (C-4′), 62.5 (C-6), 61.0 (C-6′), 56.1 (C-2), 23.5 (NHC(O)CH3), 20.8, 20.8, 20.6, 20.6, 20.5 (C(O)CH3); HRMS (ESI) m/z for C32H40N2O19: [M + Na]+ calcd 779.2123 found 779.2134.
7); 1H-NMR (400 MHz, CDCl3) δ 8.21–8.16 (2H, m, Harom), 7.10–7.04 (2H, m, Harom), 5.93 (1H, d, J = 7.5 Hz, NH), 5.71 (1H, d, J1,2 = 7.0 Hz, H-1), 5.37–5.35 (1H, m, H-4′), 5.12 (1H, dd, J1′,2′ = 7.8 Hz, J2′,3′ = 10.4 Hz, H-2′), 5.06 (1H, dd∼vt, H-4), 4.99 (1H, dd, J2′,3′ = 10.4 Hz, J3′,4′ = 3.4 Hz, H-3′), 4.64 (1H, d, J1′,2′ = 7.8 Hz, H-1′), 4.57 (1H, dd∼vt, H-3), 4.24 (1H, dd, J5,6a = 6.1 Hz, J6a/b = 12.3 Hz, H-6a), 4.19–4.07 (3H, m, H-6b, H-6′a/b), 3.98–3.88 (2H, m, H-5, H-5′), 3.67 (1H, ddd∼vdd, H-2), 2.16, 2.09, 20.8, 2.07, 2.00, 1.98 (each 3H, s, C(O)CH3), 2.02 (3H, s, NHC(O)CH3); 13C-NMR (100 MHz, CDCl3) δ 170.7, 170.4, 170.1, 170.1, 169.1, 169.1 (C(O)CH3), 161.4 (NHC(O)CH3), 143.0 (Cquaternary), 125.7, 116.5 (CHarom), 100.7 (C-1′), 96.3 (C-1), 76.0 (C-3), 72.4 (C-5), 70.9, 70.8 (C-3′, C-5′), 69.4 (C-2′), 68.5 (C-4), 66.8 (C-4′), 62.5 (C-6), 61.0 (C-6′), 56.1 (C-2), 23.5 (NHC(O)CH3), 20.8, 20.8, 20.6, 20.6, 20.5 (C(O)CH3); HRMS (ESI) m/z for C32H40N2O19: [M + Na]+ calcd 779.2123 found 779.2134.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, RF: 0.45; starting material 0.40). (2) DCM (60 mL), aqueous K2CO3 solution (60 mL, 1 M), TBAHS (1.50 g, 4.42 mmol), o-nitrophenol (620 mg, 4.46 mmol). Yield 34% (1.15 g, 1.52 mmol); colorless solid; mp 182–184 °C; [α]25D -49.6 (c 0.50 in CHCl3); RF 0.69 (heptane/acetone 3
7, RF: 0.45; starting material 0.40). (2) DCM (60 mL), aqueous K2CO3 solution (60 mL, 1 M), TBAHS (1.50 g, 4.42 mmol), o-nitrophenol (620 mg, 4.46 mmol). Yield 34% (1.15 g, 1.52 mmol); colorless solid; mp 182–184 °C; [α]25D -49.6 (c 0.50 in CHCl3); RF 0.69 (heptane/acetone 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7); 1H-NMR (400 MHz, CDCl3) δ 7.80–7.76 (1H, m, Harom), 7.54–7.49 (1H, m, Harom), 7.35–7.32 (1H, m, Harom), 7.21–7.26 (1H, m, Harom), 5.99 (1H, bs, NH), 5.62 (1H, d, J1,2 = 8.2 Hz, H-1), 5.37–5.34 (1H, m, H-4′), 5.08 (1H, dd, J1′,2′ = 7.8 Hz, J2′,3′ = 10.4 Hz, H-2′), 5.03–4.97 (1H, m, H-4), 4.98 (1H, dd, J2′,3′ = 10.4 Hz, J3′,4′ = 3.8 Hz, H-3′), 4.75 (1H, dd∼vt, H-3), 4.58 (1H, d, J1′,2′ = 7.8 Hz, H-1′), 4.25 (1H, dd, J5,6a = 5.7 Hz, J6a/b = 12.3 Hz, H-6a), 4.19 (1H, dd, J5,6b = 2.5 Hz, J6a/b = 12.3 Hz, H-6b), 4.14–4.09 (2H, m, H-6′a/b), 3.92–3.85 (2H, m, H-5, H-5′), 3.45–3.37 (1H, m, H-2), 2.15, 2.10, 2.08, 2.07, 2.06, 1.98 (each 3H, s, C(O)CH3), 2.05 (3H, s, NHC(O)CH3); 13C-NMR (100 MHz, CDCl3): δ = 171.9 (NHC(O)CH3), 170.5, 170.4, 170.2, 170.1, 169.3, 169.1 (C(O)CH3), 149.7, 141.1 (Cquaternary), 134.0, 125.1, 123.4, 119.4 (CHarom), 100.8 (C-1′), 98.4 (C-1), 76.1 (C-3), 72.4 (C-5), 71.0 (C-3′), 70.7 (C-5′), 69.4 (C-2′), 68.9 (C-4), 66.9 (C-4′), 62.3 (C-6), 61.0 (C-6′), 58.2 (C-2), 23.6 (NHC(O)CH3), 20.9, 20.8, 20.7, 20.6, 20.6, 20.5 (C(O)CH3); HRMS (ESI) m/z for C32H40N2O19: [M + Na]+ calcd 779.2123 found 779.2139.
7); 1H-NMR (400 MHz, CDCl3) δ 7.80–7.76 (1H, m, Harom), 7.54–7.49 (1H, m, Harom), 7.35–7.32 (1H, m, Harom), 7.21–7.26 (1H, m, Harom), 5.99 (1H, bs, NH), 5.62 (1H, d, J1,2 = 8.2 Hz, H-1), 5.37–5.34 (1H, m, H-4′), 5.08 (1H, dd, J1′,2′ = 7.8 Hz, J2′,3′ = 10.4 Hz, H-2′), 5.03–4.97 (1H, m, H-4), 4.98 (1H, dd, J2′,3′ = 10.4 Hz, J3′,4′ = 3.8 Hz, H-3′), 4.75 (1H, dd∼vt, H-3), 4.58 (1H, d, J1′,2′ = 7.8 Hz, H-1′), 4.25 (1H, dd, J5,6a = 5.7 Hz, J6a/b = 12.3 Hz, H-6a), 4.19 (1H, dd, J5,6b = 2.5 Hz, J6a/b = 12.3 Hz, H-6b), 4.14–4.09 (2H, m, H-6′a/b), 3.92–3.85 (2H, m, H-5, H-5′), 3.45–3.37 (1H, m, H-2), 2.15, 2.10, 2.08, 2.07, 2.06, 1.98 (each 3H, s, C(O)CH3), 2.05 (3H, s, NHC(O)CH3); 13C-NMR (100 MHz, CDCl3): δ = 171.9 (NHC(O)CH3), 170.5, 170.4, 170.2, 170.1, 169.3, 169.1 (C(O)CH3), 149.7, 141.1 (Cquaternary), 134.0, 125.1, 123.4, 119.4 (CHarom), 100.8 (C-1′), 98.4 (C-1), 76.1 (C-3), 72.4 (C-5), 71.0 (C-3′), 70.7 (C-5′), 69.4 (C-2′), 68.9 (C-4), 66.9 (C-4′), 62.3 (C-6), 61.0 (C-6′), 58.2 (C-2), 23.6 (NHC(O)CH3), 20.9, 20.8, 20.7, 20.6, 20.6, 20.5 (C(O)CH3); HRMS (ESI) m/z for C32H40N2O19: [M + Na]+ calcd 779.2123 found 779.2139.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7); 1H-NMR (400 MHz, CDCl3) δ 8.21–8.17 (2H, m, Harom), 7.10–7.05 (2H, m, Harom), 6.19 (1H, d, J = 8.8 Hz, NH), 5.40–5.38 (1H, m, H-4′), 5.30 (1H, d, J1,2 = 6.0 Hz, H-1), 5.21 (1H, dd∼vt, H-3), 5.14 (1H, dd, J1′,2′ = 7.9 Hz, J2′,3′ = 10.4 Hz, H-2′), 5.02 (1H, dd, J2′,3′ = 10.4 Hz, J3′,4′ = 3.5 Hz, H-3′), 4.51 (1H, d, J1′,2′ = 7.9 Hz, H-1′), 4.47 (1H, dd, J5,6a = 4.1 Hz, J6a/b = 12.0 Hz, H-6a), 4.43–4.37 (1H, m, H-2), 4.20–4.08 (3H, m, H-6b, H-6′a/b), 3.97–3.90 (2H, m, H-5, H-5′), 3.89–3.85 (1H, m, H-4), 2.14, 2.13, 2.10, 2.06, 2.01, 1.99 (each 3H, s, C(O)CH3), 2.02 (NC(O)CH3); 13C-NMR (100 MHz, CDCl3) δ 170.3, 170.3, 170.2, 170.1, 170.0, 169.9, 169.8 (C(O)CH3), 161.3, 142.8 (Cquaternary), 125.7, 116.4 (CHarom), 100.7 (C-1′), 97.8 (C-1), 74.0 (C-4), 72.9 (C-5), 71.0 (C-5′), 70.4 (C-3, C-3′), 69.0 (C-2′), 66.6 (C-4′), 62.5 (C-6), 60.8 (C-6′), 51.1 (C-2), 23.1 (NC(O)CH3), 20.9, 20.7, 20.6, 20.6, 20.5, 20.5 (C(O)CH3); HRMS (ESI) m/z for C32H40N2O19: [M + Na]+ calcd 779.2123 found 779.2133.
7); 1H-NMR (400 MHz, CDCl3) δ 8.21–8.17 (2H, m, Harom), 7.10–7.05 (2H, m, Harom), 6.19 (1H, d, J = 8.8 Hz, NH), 5.40–5.38 (1H, m, H-4′), 5.30 (1H, d, J1,2 = 6.0 Hz, H-1), 5.21 (1H, dd∼vt, H-3), 5.14 (1H, dd, J1′,2′ = 7.9 Hz, J2′,3′ = 10.4 Hz, H-2′), 5.02 (1H, dd, J2′,3′ = 10.4 Hz, J3′,4′ = 3.5 Hz, H-3′), 4.51 (1H, d, J1′,2′ = 7.9 Hz, H-1′), 4.47 (1H, dd, J5,6a = 4.1 Hz, J6a/b = 12.0 Hz, H-6a), 4.43–4.37 (1H, m, H-2), 4.20–4.08 (3H, m, H-6b, H-6′a/b), 3.97–3.90 (2H, m, H-5, H-5′), 3.89–3.85 (1H, m, H-4), 2.14, 2.13, 2.10, 2.06, 2.01, 1.99 (each 3H, s, C(O)CH3), 2.02 (NC(O)CH3); 13C-NMR (100 MHz, CDCl3) δ 170.3, 170.3, 170.2, 170.1, 170.0, 169.9, 169.8 (C(O)CH3), 161.3, 142.8 (Cquaternary), 125.7, 116.4 (CHarom), 100.7 (C-1′), 97.8 (C-1), 74.0 (C-4), 72.9 (C-5), 71.0 (C-5′), 70.4 (C-3, C-3′), 69.0 (C-2′), 66.6 (C-4′), 62.5 (C-6), 60.8 (C-6′), 51.1 (C-2), 23.1 (NC(O)CH3), 20.9, 20.7, 20.6, 20.6, 20.5, 20.5 (C(O)CH3); HRMS (ESI) m/z for C32H40N2O19: [M + Na]+ calcd 779.2123 found 779.2133.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, RF: 0.52; starting material 0.43). (2) DCM (60 mL), aqueous K2CO3 solution (60 mL, 1 M), TBAHS (1.50 g, 4.42 mmol), o-nitrophenol (620 mg, 4.46 mmol). Yield 44% (1.49 g, 1.97 mmol); colorless solid; mp 118 °C; [α]25D -12.0 (c 0.55 in CHCl3); RF 0.40 (heptane/acetone 3
7, RF: 0.52; starting material 0.43). (2) DCM (60 mL), aqueous K2CO3 solution (60 mL, 1 M), TBAHS (1.50 g, 4.42 mmol), o-nitrophenol (620 mg, 4.46 mmol). Yield 44% (1.49 g, 1.97 mmol); colorless solid; mp 118 °C; [α]25D -12.0 (c 0.55 in CHCl3); RF 0.40 (heptane/acetone 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7); 1H-NMR (400 MHz, CDCl3) δ 7.78 (1H, dd, Harom), 7.54–7.48 (1H, m, Harom), 7.36–7.32 (1H, m, Harom), 7.17–7.12 (1H, m, Harom), 6.22 (1H, d, J = 8.8 Hz, NH), 5.40–5.35 (2H, m, H-1, H-4′), 5.25 (1H, dd∼vt, H-3), 5.16 (1H, dd, J1′,2′ = 7.8 Hz, J2′,3′ = 10.4 Hz, H-2′), 5.03 (1H, dd, J2′,3′ = 10.4 Hz, J3′,4′ = 3.5 Hz, H-3′), 4.54 (1H, dd, J5′,6a′ = 3.8 Hz, J6a/b′ = 11.6 Hz, H-6a), 4.52 (1H, J1′,2′ = 7.8 Hz, H-1′), 4.32–4.22 (2H, m, H-2, H-6b), 4.20–4.28 (2H, m, H-6′a/b), 3.96–3.88 (3H, m, H-4, H-5, H-5′), 2.16, 2.16, 2.08, 2.04, 1.98, 1.98 (each 3H, s, C(O)CH3), 2.05 (3H, s, NC(O)CH3); 13C-NMR (100 MHz, CDCl3) δ 170.5, 170.3, 170.1, 170.0, 169.9, 169.7 (C(O)CH3), 149.2, 141.2 (Cquaternary), 133.7, 125.1, 122.9, 119.0 (CHarom), 100.6 (C-1′), 98.4 (C-1), 73.7 (C-4), 72.9 (C-5), 71.0 (C-5′), 70.5 (C-3′), 69.4 (C-3), 69.0 (C-2′), 66.6 (C-4′), 62.4 (C-6), 60.8 (C-6′), 50.9 (C-2) 23.1 (NC(O)CH3), 20.8, 20.7, 20.6, 20.6, 20.6, 20.5 (C(O)CH3); HRMS (ESI) m/z for C32H40N2O19: [M + Na]+ calcd 779.2123 found 779.2122.
7); 1H-NMR (400 MHz, CDCl3) δ 7.78 (1H, dd, Harom), 7.54–7.48 (1H, m, Harom), 7.36–7.32 (1H, m, Harom), 7.17–7.12 (1H, m, Harom), 6.22 (1H, d, J = 8.8 Hz, NH), 5.40–5.35 (2H, m, H-1, H-4′), 5.25 (1H, dd∼vt, H-3), 5.16 (1H, dd, J1′,2′ = 7.8 Hz, J2′,3′ = 10.4 Hz, H-2′), 5.03 (1H, dd, J2′,3′ = 10.4 Hz, J3′,4′ = 3.5 Hz, H-3′), 4.54 (1H, dd, J5′,6a′ = 3.8 Hz, J6a/b′ = 11.6 Hz, H-6a), 4.52 (1H, J1′,2′ = 7.8 Hz, H-1′), 4.32–4.22 (2H, m, H-2, H-6b), 4.20–4.28 (2H, m, H-6′a/b), 3.96–3.88 (3H, m, H-4, H-5, H-5′), 2.16, 2.16, 2.08, 2.04, 1.98, 1.98 (each 3H, s, C(O)CH3), 2.05 (3H, s, NC(O)CH3); 13C-NMR (100 MHz, CDCl3) δ 170.5, 170.3, 170.1, 170.0, 169.9, 169.7 (C(O)CH3), 149.2, 141.2 (Cquaternary), 133.7, 125.1, 122.9, 119.0 (CHarom), 100.6 (C-1′), 98.4 (C-1), 73.7 (C-4), 72.9 (C-5), 71.0 (C-5′), 70.5 (C-3′), 69.4 (C-3), 69.0 (C-2′), 66.6 (C-4′), 62.4 (C-6), 60.8 (C-6′), 50.9 (C-2) 23.1 (NC(O)CH3), 20.8, 20.7, 20.6, 20.6, 20.6, 20.5 (C(O)CH3); HRMS (ESI) m/z for C32H40N2O19: [M + Na]+ calcd 779.2123 found 779.2122.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, RF: 0.52; starting material 0.43). (2) DCM (17 mL), aqueous K2CO3 solution (17 mL, 1 M), TBAHS (520 mg, 1.53 mmol), 5-bromo-4-chloro-indoxylic acid allyl ester (500 mg, 1.51 mmol). Yield 46% (635 mg, 0.670 mmol); colorless solid; mp 152–155 °C; [α]23D -24.2 (c 0.5, CHCl3); RF 0.39 (hexane/acetone 1
7, RF: 0.52; starting material 0.43). (2) DCM (17 mL), aqueous K2CO3 solution (17 mL, 1 M), TBAHS (520 mg, 1.53 mmol), 5-bromo-4-chloro-indoxylic acid allyl ester (500 mg, 1.51 mmol). Yield 46% (635 mg, 0.670 mmol); colorless solid; mp 152–155 °C; [α]23D -24.2 (c 0.5, CHCl3); RF 0.39 (hexane/acetone 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H-NMR (400 MHz, CDCl3) δ 8.69 (1H, s, NH), 7.49 (1H, d, J = 8.9 Hz, Harom), 7.10 (1H, d, J = 8.9 Hz, Harom), 6.37 (1H, d, J = 7.6 Hz, C2–NH), 6.09–6.01 (1H, m, –O–CH2–CH
1); 1H-NMR (400 MHz, CDCl3) δ 8.69 (1H, s, NH), 7.49 (1H, d, J = 8.9 Hz, Harom), 7.10 (1H, d, J = 8.9 Hz, Harom), 6.37 (1H, d, J = 7.6 Hz, C2–NH), 6.09–6.01 (1H, m, –O–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.47–5.43 (1H, m, O–CH2–CH
CH2), 5.47–5.43 (1H, m, O–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2a), 5.40–5.35 (3H, m, H-1, H-4′, O–CH2–CH
CH2a), 5.40–5.35 (3H, m, H-1, H-4′, O–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2b), 5.09 (1H, dd, J1′,2′ = 7.9 Hz, J2′,3′ = 10.4 Hz, H-2′), 5.03–4.98 (2H, m, H-3′, H-4), 4.70 (1H, d, J1′,2′ = 7.9 Hz, H-1′), 4.90–4.83 (2H, m, O–CH2–CH
CH2b), 5.09 (1H, dd, J1′,2′ = 7.9 Hz, J2′,3′ = 10.4 Hz, H-2′), 5.03–4.98 (2H, m, H-3′, H-4), 4.70 (1H, d, J1′,2′ = 7.9 Hz, H-1′), 4.90–4.83 (2H, m, O–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.35 (1H, dd∼vt, H-3), 4.10 (1H, dd, J5,6a = 4.9 Hz, J6a,6b = 12.1 Hz, H-6a), 4.00 (1H, dd, J5,6b = 3.0 Hz, J6a,6b = 12.1 Hz, H-6b), 3.99–3.94 (1H, m, H-2), 3.92–3.88 (1H, m, H-5′), 3.62 (1H, ddd, J4,5 = 9.4 Hz, J5,6a = 4.9 Hz, J5,6b = 3.0 Hz, H-5), 2.15, 2.13, 2.06, 2.03, 1.97, 1.95 (each 3H, s, C(O)CH3), 1.99 (3H, s, NHC(O)CH3); 13C-NMR (100 MHz, CDCl3) δ 171.2, 170.8, 170.6, 170.4, 169.6, 169.4 (C(O)CH3), 159.9 (C(O)O–CH2–), 140.3, 133.5 (Cquaternary), 131.7 (O–CH2–CH
CH2), 4.35 (1H, dd∼vt, H-3), 4.10 (1H, dd, J5,6a = 4.9 Hz, J6a,6b = 12.1 Hz, H-6a), 4.00 (1H, dd, J5,6b = 3.0 Hz, J6a,6b = 12.1 Hz, H-6b), 3.99–3.94 (1H, m, H-2), 3.92–3.88 (1H, m, H-5′), 3.62 (1H, ddd, J4,5 = 9.4 Hz, J5,6a = 4.9 Hz, J5,6b = 3.0 Hz, H-5), 2.15, 2.13, 2.06, 2.03, 1.97, 1.95 (each 3H, s, C(O)CH3), 1.99 (3H, s, NHC(O)CH3); 13C-NMR (100 MHz, CDCl3) δ 171.2, 170.8, 170.6, 170.4, 169.6, 169.4 (C(O)CH3), 159.9 (C(O)O–CH2–), 140.3, 133.5 (Cquaternary), 131.7 (O–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 131.7 (CHarom), 127.3, 120.9 (Cquaternary), 119.9 (O–CH2–CH
CH2), 131.7 (CHarom), 127.3, 120.9 (Cquaternary), 119.9 (O–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 116.9, 115.7 (Cquaternary), 111.7 (CHarom), 102.1 (C-1), 100.9 (C-1′), 77.6 (C-3), 72.3 (C-5), 71.4, 69.4 (C-3′, C-4), 70.6 (C-5′), 69.3 (C-2′), 67.1 (C-4′), 66.1 (O–CH2–CH
CH2), 116.9, 115.7 (Cquaternary), 111.7 (CHarom), 102.1 (C-1), 100.9 (C-1′), 77.6 (C-3), 72.3 (C-5), 71.4, 69.4 (C-3′, C-4), 70.6 (C-5′), 69.3 (C-2′), 67.1 (C-4′), 66.1 (O–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 62.1 (C-6), 61.1 (C-6′), 57.3 (C-2), 23.7 (NHC(O)CH3), 21.1, 20.9, 20.8, 20.8, 20.8, 20.7 (C(O)CH3); HRMS (ESI) m/z for C38H44BrClN2O19: [M + Na]+ calcd 969.1308 found 969.1293.
CH2), 62.1 (C-6), 61.1 (C-6′), 57.3 (C-2), 23.7 (NHC(O)CH3), 21.1, 20.9, 20.8, 20.8, 20.8, 20.7 (C(O)CH3); HRMS (ESI) m/z for C38H44BrClN2O19: [M + Na]+ calcd 969.1308 found 969.1293.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, RF: 0.52; starting material 0.43). (2) DCM (15 mL), aqueous K2CO3 solution (15 mL, 1 M), TBAHS (414 mg, 1.22 mmol), 5-bromo-4-chloro-indoxylic acid allyl ester (400 mg, 1.21 mmol). Yield 45% (562 mg, 0.593 mmol); colorless solid; mp 149–152 °C; [α]23D -7.6 (c 0.5 in CHCl3); RF 0.41 (hexane/acetone 1
7, RF: 0.52; starting material 0.43). (2) DCM (15 mL), aqueous K2CO3 solution (15 mL, 1 M), TBAHS (414 mg, 1.22 mmol), 5-bromo-4-chloro-indoxylic acid allyl ester (400 mg, 1.21 mmol). Yield 45% (562 mg, 0.593 mmol); colorless solid; mp 149–152 °C; [α]23D -7.6 (c 0.5 in CHCl3); RF 0.41 (hexane/acetone 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H-NMR (400 MHz, CDCl3) δ 8.64 (1H, s, NH), 7.48 (1H, d, J = 8.8 Hz, Harom), 7.09 (1H, d, J = 8.8 Hz, Harom), 6.58 (1H, d, J = 8.6 Hz, C2–NH), 6.08–6.01 (1H, m, –O–CH2–CH
1); 1H-NMR (400 MHz, CDCl3) δ 8.64 (1H, s, NH), 7.48 (1H, d, J = 8.8 Hz, Harom), 7.09 (1H, d, J = 8.8 Hz, Harom), 6.58 (1H, d, J = 8.6 Hz, C2–NH), 6.08–6.01 (1H, m, –O–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.49–5.45 (1H, m, O–CH2–CH = CH2a), 5.41–5.38 (1H, m, O–CH2–CH
CH2), 5.49–5.45 (1H, m, O–CH2–CH = CH2a), 5.41–5.38 (1H, m, O–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2b), 5.35–5.33 (1H, m, H-4′), 5.15–5.07 (3H, m, H-1, H-2′, H-3), 4.96 (1H, dd, J2′,3′ = 10.5 Hz, J3′,4′ = 10.5 Hz, H-3′), 4.89–4.81 (2H, m, O–CH2–CH
CH2b), 5.35–5.33 (1H, m, H-4′), 5.15–5.07 (3H, m, H-1, H-2′, H-3), 4.96 (1H, dd, J2′,3′ = 10.5 Hz, J3′,4′ = 10.5 Hz, H-3′), 4.89–4.81 (2H, m, O–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.53 (1H, d, J1′,2′ = 7.6 Hz, H-1′), 4.46–4.41 (1H, m, H-2), 4.31 (1H, dd, J5,6a = 2.4 Hz, J6a,6b = 11.8 Hz, H-6a), 4.15–4.09 (2H, m, H-6′a/b), 3.93 (1H, dd∼vt, H-4), 3.90–3.86 (1H, m, H-5′), 3.46–3.42 (1H, m, H-5), 2.14, 2.12, 2.06, 1.98 (each 3H, s, C(O)CH3), 1.99 (3H, s, NHC(O)CH3), 1.95 (6H, s, 2C(O)CH3); 13C-NMR (100 MHz, CDCl3) δ 170.7, 170.6, 170.3, 170.2, 170.1, 170.0, 169.1 (C(O)CH3), 159.4 (C(O)O–CH2–), 141.3, 133.2 (Cquaternary), 131.4 (O–CH2–CH
CH2), 4.53 (1H, d, J1′,2′ = 7.6 Hz, H-1′), 4.46–4.41 (1H, m, H-2), 4.31 (1H, dd, J5,6a = 2.4 Hz, J6a,6b = 11.8 Hz, H-6a), 4.15–4.09 (2H, m, H-6′a/b), 3.93 (1H, dd∼vt, H-4), 3.90–3.86 (1H, m, H-5′), 3.46–3.42 (1H, m, H-5), 2.14, 2.12, 2.06, 1.98 (each 3H, s, C(O)CH3), 1.99 (3H, s, NHC(O)CH3), 1.95 (6H, s, 2C(O)CH3); 13C-NMR (100 MHz, CDCl3) δ 170.7, 170.6, 170.3, 170.2, 170.1, 170.0, 169.1 (C(O)CH3), 159.4 (C(O)O–CH2–), 141.3, 133.2 (Cquaternary), 131.4 (O–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 131.0 (CHarom), 127.4, 120.7 (Cquaternary), 120.1 (O–CH2–CH
CH2), 131.0 (CHarom), 127.4, 120.7 (Cquaternary), 120.1 (O–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 116.1, 115.7 (Cquaternary), 111.5 (CHarom), 103.2 (C-1), 101.2 (C-1′), 74.1 (C-3), 73.3 (C-4), 72.8 (C-5), 70.9 (C-3′), 70.7 (C-5′), 69.2 (C-2′), 66.6 (C-4′), 66.0 (O–CH2–CH
CH2), 116.1, 115.7 (Cquaternary), 111.5 (CHarom), 103.2 (C-1), 101.2 (C-1′), 74.1 (C-3), 73.3 (C-4), 72.8 (C-5), 70.9 (C-3′), 70.7 (C-5′), 69.2 (C-2′), 66.6 (C-4′), 66.0 (O–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 61.2 (C-6), 60.8 (C-6′), 54.3 (C-2), 23.3 (NHC(O)CH3), 20.9, 20.6, 20.6, 20.6, 20.5 (C(O)CH3); HRMS (ESI) m/z for C38H44BrClN2O19: [M + Na]+ calcd 969.1308 found 969.1288.
CH2), 61.2 (C-6), 60.8 (C-6′), 54.3 (C-2), 23.3 (NHC(O)CH3), 20.9, 20.6, 20.6, 20.6, 20.5 (C(O)CH3); HRMS (ESI) m/z for C38H44BrClN2O19: [M + Na]+ calcd 969.1308 found 969.1288.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H-NMR (400 MHz, CDCl3) δ 8.25 (1H, d, J = 8.9 Hz, Harom), 7.55 (1H, d, J = 8.9 Hz, Harom), 7.29 (1H, s,
1); 1H-NMR (400 MHz, CDCl3) δ 8.25 (1H, d, J = 8.9 Hz, Harom), 7.55 (1H, d, J = 8.9 Hz, Harom), 7.29 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–N), 5.87 (1H, d, J = 7.8 Hz, C2–NH), 5.41 (1H, d, J1,2 = 7.9 Hz, H-1), 5.37–5.35 (1H, m, H-4′), 5.10 (1H, dd, J1′,2′ = 7.8 Hz, J2′,2′ = 10.5 Hz, H-2′), 5.03 (1H, dd∼vt, H-4), 4.99 (1H, dd, J2′,3′ = 10.5 Hz, J3′,4′ = 3.4 Hz, H-3′), 4.66–4.59 (1H, m, H-3), 4.62 (1H, d, J1′,2′ = 7.8 Hz, H-1′), 4.37 (1H, dd, J5,6a = 2.7 Hz, J6a/b = 12.4 Hz, H-6a), 4.18 (1H, dd, J5,6b = 5.5 Hz, J6a/b = 12.4 Hz, H-6b), 4.14–4.08 (2H, m, H-6′a/b), 3.92–3.84 (2H, m, H-5, H-5′), 3.66–3.57 (1H, m, H-2), 2.60, 2.16, 2.09, 2.09, 2.08, 2.06, 2.05, 1.98 (each 3H, s, C(O)CH3); 13C-NMR (100 MHz, CDCl3) δ 171.1, 170.5, 170.4, 170.2, 170.1, 169.3, 169.1, 168.2 (C(O)CH3), 140.0, 133.4 (Cquaternary), 130.5 (CHarom), 124.7, 122.6, 118.4 (Cquaternary), 116.3 (CHarom), 112.4 (
CH–N), 5.87 (1H, d, J = 7.8 Hz, C2–NH), 5.41 (1H, d, J1,2 = 7.9 Hz, H-1), 5.37–5.35 (1H, m, H-4′), 5.10 (1H, dd, J1′,2′ = 7.8 Hz, J2′,2′ = 10.5 Hz, H-2′), 5.03 (1H, dd∼vt, H-4), 4.99 (1H, dd, J2′,3′ = 10.5 Hz, J3′,4′ = 3.4 Hz, H-3′), 4.66–4.59 (1H, m, H-3), 4.62 (1H, d, J1′,2′ = 7.8 Hz, H-1′), 4.37 (1H, dd, J5,6a = 2.7 Hz, J6a/b = 12.4 Hz, H-6a), 4.18 (1H, dd, J5,6b = 5.5 Hz, J6a/b = 12.4 Hz, H-6b), 4.14–4.08 (2H, m, H-6′a/b), 3.92–3.84 (2H, m, H-5, H-5′), 3.66–3.57 (1H, m, H-2), 2.60, 2.16, 2.09, 2.09, 2.08, 2.06, 2.05, 1.98 (each 3H, s, C(O)CH3); 13C-NMR (100 MHz, CDCl3) δ 171.1, 170.5, 170.4, 170.2, 170.1, 169.3, 169.1, 168.2 (C(O)CH3), 140.0, 133.4 (Cquaternary), 130.5 (CHarom), 124.7, 122.6, 118.4 (Cquaternary), 116.3 (CHarom), 112.4 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–N), 100.8 (C-1′), 99.4 (C-1), 76.2 (C-3), 72.6 (C-5), 71.0 (C-5′), 70.7 (C-3′), 69.4, 69.0 (C-2′, C-4), 66.9 (C-4′), 62.4 (C-6), 61.0 (C-6′), 57.4 (C-2), 23.8, 23.8, 20.9, 20.8, 20.8, 20.7, 20.6, 20.5 (C(O)CH3); HRMS (ESI) m/z for C36H42BrClN2O18: [M + Na]+ calcd 927.1202 found 927.1194.
CH–N), 100.8 (C-1′), 99.4 (C-1), 76.2 (C-3), 72.6 (C-5), 71.0 (C-5′), 70.7 (C-3′), 69.4, 69.0 (C-2′, C-4), 66.9 (C-4′), 62.4 (C-6), 61.0 (C-6′), 57.4 (C-2), 23.8, 23.8, 20.9, 20.8, 20.8, 20.7, 20.6, 20.5 (C(O)CH3); HRMS (ESI) m/z for C36H42BrClN2O18: [M + Na]+ calcd 927.1202 found 927.1194.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H-NMR (400 MHz, CDCl3) δ 8.17 (1H, d, J = 8.9 Hz, Harom), 7.54 (1H, d, J = 8.9 Hz, Harom), 7.44 (1H, s, =CH–N), 6.19 (1H, d, J = 9.1 Hz, C2–NH), 5.41–5.38 (1H, m, H-4′), 5.22–5.15 (2H, m, H-3, H-2′), 5.11 (1H, d, J1,2 = 5.4 Hz, H-1), 5.05 (1H, dd, J2′,3′ = 10.4 Hz, J3′,4′ = 3.3 Hz, H-3′), 4.81 (1H, dd, J5,6a = 4.7 Hz, J6a/b = 11.8 Hz, H-6a), 4.57 (1H, d, J1′,2′ = 8.0 Hz, H-1′), 4.44–4.39 (1H, m, H-2), 4.24 (1H, dd, J5,6b = 5.0 Hz, J6a/b = 11.8 Hz, H-6b), 4.19 (1H, dd, J5′,6′a = 6.2 Hz, J6′a/b = 11.3 Hz, H-6′a), 4.12 (1H, dd, J5′,6′b = 7.1 Hz, J6′a/b = 11.3 Hz, H-6′b), 3.97–3.87 (3H, m, H-4, H-5, H-5′), 2.17, 2.09, 1.99 (each 3H, s, C(O)CH3), 2.08, 2.06 (each 6H, s, 2C(O)CH3); 13C-NMR (100 MHz, CDCl3) δ 170.4, 170.4, 170.3, 170.1, 170.0, 170.0, 169.7, 168.6 (C(O)CH3), 139.4, 133.5 (Cquaternary), 130.4 (CHarom), 118.3 (Cquaternary), 116.3 (CHarom), 111.8 (
1); 1H-NMR (400 MHz, CDCl3) δ 8.17 (1H, d, J = 8.9 Hz, Harom), 7.54 (1H, d, J = 8.9 Hz, Harom), 7.44 (1H, s, =CH–N), 6.19 (1H, d, J = 9.1 Hz, C2–NH), 5.41–5.38 (1H, m, H-4′), 5.22–5.15 (2H, m, H-3, H-2′), 5.11 (1H, d, J1,2 = 5.4 Hz, H-1), 5.05 (1H, dd, J2′,3′ = 10.4 Hz, J3′,4′ = 3.3 Hz, H-3′), 4.81 (1H, dd, J5,6a = 4.7 Hz, J6a/b = 11.8 Hz, H-6a), 4.57 (1H, d, J1′,2′ = 8.0 Hz, H-1′), 4.44–4.39 (1H, m, H-2), 4.24 (1H, dd, J5,6b = 5.0 Hz, J6a/b = 11.8 Hz, H-6b), 4.19 (1H, dd, J5′,6′a = 6.2 Hz, J6′a/b = 11.3 Hz, H-6′a), 4.12 (1H, dd, J5′,6′b = 7.1 Hz, J6′a/b = 11.3 Hz, H-6′b), 3.97–3.87 (3H, m, H-4, H-5, H-5′), 2.17, 2.09, 1.99 (each 3H, s, C(O)CH3), 2.08, 2.06 (each 6H, s, 2C(O)CH3); 13C-NMR (100 MHz, CDCl3) δ 170.4, 170.4, 170.3, 170.1, 170.0, 170.0, 169.7, 168.6 (C(O)CH3), 139.4, 133.5 (Cquaternary), 130.4 (CHarom), 118.3 (Cquaternary), 116.3 (CHarom), 111.8 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–N), 100.8 (C-1′), 99.7 (C-1), 73.8, 73.1 (C-4, C-5), 71.0 (C-5′), 70.5 (C-3′), 70.0 (C-3), 69.1 (C-2′), 66.6 (C-4′), 62.3 (C-6), 60.8 (C-6′), 50.3 (C-2), 23.9, 23.2, 20.8, 20.7, 20.6, 20.5 (C(O)CH3); HRMS (ESI) m/z for C36H42BrClN2O18: [M + Na]+ calcd 927.1202 found 927.1187.
CH–N), 100.8 (C-1′), 99.7 (C-1), 73.8, 73.1 (C-4, C-5), 71.0 (C-5′), 70.5 (C-3′), 70.0 (C-3), 69.1 (C-2′), 66.6 (C-4′), 62.3 (C-6), 60.8 (C-6′), 50.3 (C-2), 23.9, 23.2, 20.8, 20.7, 20.6, 20.5 (C(O)CH3); HRMS (ESI) m/z for C36H42BrClN2O18: [M + Na]+ calcd 927.1202 found 927.1187.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–N), 4.87 (1H, d, J1,2 = 8.5 Hz, H-1), 4.83–4.70 (3H, m, 3 OH), 4.66 (1H, bs, OH), 4.50 (1H, bs, OH), 4.33 (1H, bs, OH), 4.15 (1H, d, J1′,2′ = 6.6 Hz, H-1′), 3.85–3.75 (2H, m, H-2, H-6a), 3.66–3.58 (2H, m, H-3, H-4′), 3.57–3.49 (3H, m, H-6b, H-6′a/b), 3.49–3.31 (5H, m, H-2′, H-3′, H-4, H-5, H-5′), 1.82 (3H, s, NHC(O)CH3); 13C-NMR (100 MHz, DMSO-d6) δ 169.9 (C(O)CH3), 132.9 (Cquaternary), 125.5 (CHarom), 122.9, 117.6 (Cquaternary), 114.0, 112.3 (CHarom, =CH–N), 111.7 (Cquaternary), 104.0 (C-1′), 101.7 (C-1), 84.6 (C-3), 76.8, 75.8 (C-5, C-5′), 72.9, 68.9 (C-3′, C-4), 70.6 (C-2′), 68.2 (C-4′), 60.8, 60.6 (C-6, C-6′), 54.2 (C-2), 23.1 (C(O)CH3) ppm.
CH–N), 4.87 (1H, d, J1,2 = 8.5 Hz, H-1), 4.83–4.70 (3H, m, 3 OH), 4.66 (1H, bs, OH), 4.50 (1H, bs, OH), 4.33 (1H, bs, OH), 4.15 (1H, d, J1′,2′ = 6.6 Hz, H-1′), 3.85–3.75 (2H, m, H-2, H-6a), 3.66–3.58 (2H, m, H-3, H-4′), 3.57–3.49 (3H, m, H-6b, H-6′a/b), 3.49–3.31 (5H, m, H-2′, H-3′, H-4, H-5, H-5′), 1.82 (3H, s, NHC(O)CH3); 13C-NMR (100 MHz, DMSO-d6) δ 169.9 (C(O)CH3), 132.9 (Cquaternary), 125.5 (CHarom), 122.9, 117.6 (Cquaternary), 114.0, 112.3 (CHarom, =CH–N), 111.7 (Cquaternary), 104.0 (C-1′), 101.7 (C-1), 84.6 (C-3), 76.8, 75.8 (C-5, C-5′), 72.9, 68.9 (C-3′, C-4), 70.6 (C-2′), 68.2 (C-4′), 60.8, 60.6 (C-6, C-6′), 54.2 (C-2), 23.1 (C(O)CH3) ppm.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–N), 112.3 (CHarom), 111.6 (Cquaternary), 104.0 (C-1′), 102.0 (C-1), 81.4 (C-4), 75.6, 75.4 (C-5, C-5′), 73.2, 70.6 (C-2′, C-3′), 72.3 (C-3), 68.2 (C-4′), 60.5, 60.4 (C-6, C-6′), 54.6 (C-2), 23.1 (C(O)CH3) ppm.
CH–N), 112.3 (CHarom), 111.6 (Cquaternary), 104.0 (C-1′), 102.0 (C-1), 81.4 (C-4), 75.6, 75.4 (C-5, C-5′), 73.2, 70.6 (C-2′, C-3′), 72.3 (C-3), 68.2 (C-4′), 60.5, 60.4 (C-6, C-6′), 54.6 (C-2), 23.1 (C(O)CH3) ppm.Acknowledgements
Support of this work by Glycom A/S, Copenhagen, Denmark, is gratefully acknowledged.References
- R. M. Erney, W. T. Malone, M. B. Skelding, A. A. Marcon, K. M. Kleman-Leyer, M. L. O'Ryan, G. Ruiz-Palacios, M. D. Hilty, L. K. L. K. Pickering and P. A. Prieto, J. Pediatr. Gastroenterol. Nutr., 2000, 30, 181–192 CrossRef CAS PubMed.
- B. La Ferla, D. Prosperi, L. Lay, G. Russo and L. Panza, Carbohydr. Res., 2002, 337, 1333–1342 CrossRef CAS.
- J. L. Magnani, Arch. Biochem. Biophys., 2004, 426, 122–131 CrossRef CAS PubMed.
- K. Lau, H. Yu, V. Thon, Z. Khedri, M. E. Leon, B. K. Tran and X. Chen, Org. Biomol. Chem., 2011, 9, 2784–2789 CAS.
- S. C. Stocks, M. Albrechtsen and M. A. Kerr, Biochem. J., 1990, 268, 275–280 CAS.
- R. S. Loka, C. M. Sadek, N. A. Romaniuk and C. W. Cairo, Bioconjugate Chem., 2010, 21, 1842–1849 CrossRef CAS PubMed.
- D. Garrido, D. C. Dallas and D. A. Mills, Microbiology, 2013, 159, 649–664 CrossRef CAS PubMed.
- J. A. Kiernan, Biotech. Histochem., 2007, 82, 73–103 CrossRef CAS PubMed.
- J. H. Miller, Experiments in Molecular Genetics, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1972 Search PubMed.
- D. Mahrun, Clin. Chim. Acta, 1976, 73, 453–461 CrossRef.
- S. Tokutake, N. Yamaji and M. Kato, Chem. Pharm. Bull., 1990, 38, 13–18 CrossRef CAS.
- K. Clinch, G. B. Evans, R. H. Furneaux, P. M. Rendle, P. L. Rhodes, A. M. Roberton, D. I. Rosendale, P. C. Tyler and D. P. Wright, Carbohydr. Res., 2002, 337, 1095–1111 CrossRef CAS.
- G. Zemplén, Ber. Dtsch. Chem. Ges. B, 1926, 59B, 1254–1266 CrossRef.
- S. Böttcher, M. Hederos, E. Champion, G. Dekany and J. Thiem, Org. Lett., 2013, 15, 3766–3769 CrossRef PubMed.
- S. Böttcher and J. Thiem, Eur. J. Org. Chem., 2014, 564–574 CrossRef.
- H. Kunz and H. Waldmann, Angew. Chem., Int. Ed. Engl., 1984, 23, 71–72 CrossRef.
- S. S. Rana, J. J. Barlow and K. L. Matta, Carbohydr. Res., 1983, 113, 257–271 CrossRef CAS.
- H. Ohi, S. Kubota and T. Usui, Carbohydr. Res., 1993, 244, 315–323 Search PubMed.
- K. L. Matta and J. J. Barlow, Carbohydr. Res., 1975, 75, 299–304 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: NMR data for all new compounds. See DOI: 10.1039/c3ra47128d |
| This journal is © The Royal Society of Chemistry 2014 |