Synthesis and characterization of nanoporous Bi3NbO7 films: application to photoelectrochemical water splitting†
Abstract
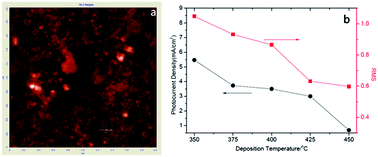
Nanostructured Bi3NbO7 films were successfully prepared via an ultrasonic spray pyrolysis method by using Bi(NO3)3·5H2O and Nb2O5 as precursors. The as-prepared films were systematically characterized by X-ray diffraction, Atomic Force Microscopy (AFM), field emission scanning electron microscopy (FESEM), UV-vis absorption spectra and X-ray photoelectron spectroscopy. The characterization results revealed that the nanostructured Bi3NbO7 possessed a cubic structure, nanoporous morphology and a visible light absorption with an optical band gap of about 2.8 eV. Moreover, electrochemical and photoelectrochemical measurements were carried out and a maximum photocurrent density of 2.8 μA cm−2 at the potential of 0.7 V vs. SCE was obtained for the Bi3NbO7 film deposited at 350 °C. The improvement of the film photoelectrochemical properties was contributed by novel nanoporous morphology that supply sufficient electrode–solution contact area. By addition of methanol into the solution, the photocurrent increased by 60%. The photoelectrochemical results reveal that the prepared films have the potential for hydrogen production via splitting water.

 Please wait while we load your content...
Please wait while we load your content...