Theoretical and experimental determination of the number of water molecules breaking the structure of a glycine-based ionic liquid†
Abstract
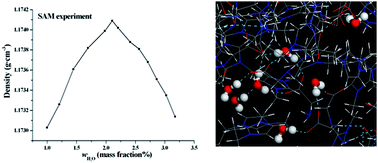
The effects of water on the structures of the amino acid ionic liquid (IL) 1-ethyl-3-methylimidazolium glycine ([emim][Gly]) are explored by a classical simulation method. The density and surface tension of the [emim][Gly]–H2O mixture are experimentally studied by a standard addition method. Simulation and experiment show that the density of the [emim][Gly]–H2O mixture reaches the maximum at 2–4 mass% water. Different analysis tools, including radial distribution, an interstice model, and molecular intrinsic characteristic contours are used to describe the structural modifications of the [emim][Gly] and ionic aggregates as a function of the solution concentration. At xw < 0.33 (mole fraction), the isolated water and dimer are located at the interstices formed between the ions, do not modify the network of ILs, and slightly strengthen the interactions between the cation and anion. Consequently, a turnover in the evolution of the IL structures and properties ensues. At 0.33 < xw < 0.50, the formation of relatively large water clusters, such as trimers and tetramers, leads to interstice distention, breakage of the cation–anion network, and gradual loosening of the interactions. With a further increased water concentration, a bicontinuous phase is generated and ionic clusters disperse in a continuous water phase. The size and morphology of the water aggregates are evaluated and analyzed by several statistical functions.

 Please wait while we load your content...
Please wait while we load your content...