Fluorescent cyclodextrin carriers for a water soluble ZnII pyrazinoporphyrazine octacation with photosensitizer potential†
Abstract
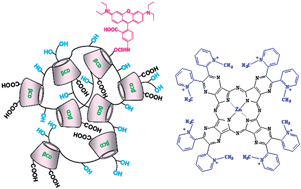
A nitro-benzofurazan-triazolyl carboxymethylated β-cyclodextrin (NBFT-CMβCyD) and an oligomer of carboxymethyl β-cyclodextrin (sodium salt), crosslinked with epichlorohydrin and labeled with rhodaminyl groups (pβCyD-Rh), exhibit very high affinity in aqueous solution for octacationic photosensitizer [(CH3)8LZn]8+ neutralized by I− ions (L = tetrakis-2,3-[5,6-di(2-(pyridiniumyl)pyrazino]porphyrazinato dianion). The photosensitizer (PS) forms complexes with 1 : 2 and 2 : 2 CyD:PS stoichiometry, which were characterized as binding constants and UV-Vis absorption and fluorescence properties. The self-association tendency of [(CH3)8LZn]8+, leading to a monomer–dimer equilibrium shift towards the dimer even at very low concentrations (≈10−6 M), is not contrasted by either NBFT-CMβCyD or the pβCyD-Rh oligomer, both of which completely convert the [(CH3)8LZn]8+ monomer fraction to the dimer form in the bound state. Quenching of fluorescence observed in [(CH3)8LZn]8+ upon binding with either hosts is consistent with the conversion of the monomer to the negligibly fluorescent dimer. The complexes formed with the CMβCyD units of the pβCyD-Rh oligomer have average association constants, which are larger by 6–7 orders of magnitude than those with the CMβCyD monomer in the NBFT-labeled derivative.

 Please wait while we load your content...
Please wait while we load your content...