A dual-chamber reactor to assess the saccharification capability of the cellulytic microflora from straw waste†
Abstract
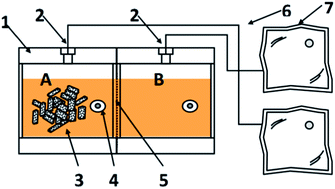
An enriched and serially diluted method was developed to screen efficient consortia to degrade straw for reducing sugar accumulation. The consortium MX with highest saccharification efficiency was selected. Then a dual-chamber reactor was designed to evaluate the saccharification capability of the screened target functional consortium.

 Please wait while we load your content...
Please wait while we load your content...