Microemulsion-like aggregation behaviour of an LCST-type ionic liquid in water
Abstract
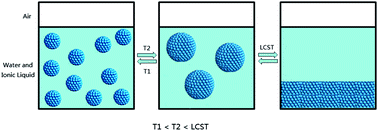
The ionic liquid (IL), tetrabutylphosphonium trifluoroacetate ([P4444][CF3COO]) showed a low critical solution temperature (LCST)-type phase transition in water. It was found that [P4444][CF3COO] molecules can form some kind of long-living aggregates in aqueous solution under certain conditions before the phase separation. These aggregates displayed the characteristic properties of microemulsions, although no surfactants are used. For instance, the aggregate droplet size can be adjusted by temperature and concentration, which behaves like the swelling phenomenon of microemulsions; the formed aggregates showed beneficial solubilization capacity for apolar substances; in addition, a tunable micropolarity in the aggregates ![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/char_0073_0332.gif)
![[o with combining low line]](https://www.rsc.org/images/entities/char_006f_0332.gif)
![[b with combining low line]](https://www.rsc.org/images/entities/char_0062_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/char_0073_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif)
![[v with combining low line]](https://www.rsc.org/images/entities/char_0076_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[d with combining low line]](https://www.rsc.org/images/entities/char_0064_0332.gif)
![[b with combining low line]](https://www.rsc.org/images/entities/char_0062_0332.gif)
![[y with combining low line]](https://www.rsc.org/images/entities/char_0079_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/char_0074_0332.gif)
![[h with combining low line]](https://www.rsc.org/images/entities/char_0068_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[d with combining low line]](https://www.rsc.org/images/entities/char_0064_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/char_0074_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[c with combining low line]](https://www.rsc.org/images/entities/char_0063_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/char_0074_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[o with combining low line]](https://www.rsc.org/images/entities/char_006f_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/char_006e_0332.gif)
![[o with combining low line]](https://www.rsc.org/images/entities/char_006f_0332.gif)
![[f with combining low line]](https://www.rsc.org/images/entities/char_0066_0332.gif)
![[U with combining low line]](https://www.rsc.org/images/entities/char_0055_0332.gif)
![[V with combining low line]](https://www.rsc.org/images/entities/char_0056_0332.gif) -Vis measurements. These are by far the simplest aggregates having the microemulsion characteristics. In nature, these microemulsion-like aggregates have mesoscopic phase separation, which is the intermediate state for macroscopic phase separation. This special system can be regarded as surfactant-free microemulsion-like aggregates and should be an effective platform to provide novel extraction or separation media.
-Vis measurements. These are by far the simplest aggregates having the microemulsion characteristics. In nature, these microemulsion-like aggregates have mesoscopic phase separation, which is the intermediate state for macroscopic phase separation. This special system can be regarded as surfactant-free microemulsion-like aggregates and should be an effective platform to provide novel extraction or separation media.

 Please wait while we load your content...
Please wait while we load your content...