One-pot synthesis of ultrafine ZnFe2O4 nanocrystals anchored on graphene for high-performance Li and Li-ion batteries†
Jian Xie*ab,
Wentao Songab,
Gaoshao Caob,
Tiejun Zhua,
Xinbing Zhao*ab and
Shichao Zhangc
aState Key Laboratory of Silicon Materials, Department of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: xiejian1977@zju.edu.cn; zhaoxb@zju.edu.cn; Fax: +86-571-87951451; Tel: +86-571-87951451
bKey Laboratory of Advanced Materials and Applications for Batteries of Zhejiang Province, China
cSchool of Materials Science and Engineering, Beijing University of Aeronautics and Astronautics, Beijing 100191, China
First published on 9th January 2014
Abstract
A ZnFe2O4-nanocrystals/graphene-nanosheets (ZnFe2O4/G) nanohybrid has been prepared by a facile in situ hydrothermal route using Zn(NO3)2·6H2O, Fe(NO3)3·6H2O and graphite oxide (GO) as the precursors. Ultrafine ZnFe2O4 nanocrystals (below 10 nm) are confined by the few-layer graphene sheets reduced from GO, forming a unique sheet-like hybrid. In this structure, the direct restacking of the hydrophobic graphene sheets is refrained by loading ZnFe2O4 nanocrystals as the spacers and the aggregation of ZnFe2O4 nanocrystals is inhibited by the dispersing and confining effects of the graphene sheets. ZnFe2O4/G shows excellent rate capability and high-rate cycling stability for lithium storage. It also shows a high capacity when used as an anode for a ZnFe2O4/G–LiFePO4/C full cell.
1. Introduction
Rechargeable Li-ion batteries (LIBs) are now considered as potential power sources for electric vehicles (EVs) and hybrid electric vehicles (HEVs). In order to meet the increasing requirements for EV and HEV applications, great attention has been paid to design new anode materials that can bring about improvement in energy density, cycle life, cost, and safety.1–3 In the past decade, an intensive research effort has been focused on transition metal oxides (TMOs) since the first report by Poizot et al.4 that reversible conversion reactions could occur for some TMOs at room temperature. Although the issue of rapid capacity fade of TMOs can be partly solved by using nanostructured materials,5,6 the long-term cycling stability, especially at high current rates, is not satisfactory yet due to the low electronic conductivity and particles aggregation upon repeated cycling. Recently, an effective solution to this problem has been provided by using the two-dimensional (2D) carbon allotrope, graphene,7 as the matrix due to its unique characteristics, such as large specific surface area,8 high electrical conductivity,9 and huge mechanical strength.10In the TMOs family, iron oxides have received a special interest because of their low cost, abundant iron resource, and environmental friendliness. Magnetite Fe3O4, a typical iron oxide, can deliver a capacity as high as 925 mA h g−1 with the conversion reaction: Fe3O4 + 8Li ↔ 4Li2O + 3Fe.11 Previous research on Fe3O4 has showed that both rate capability and cycling stability could be obviously enhanced by using graphene as the support.12–16 The improvement in electrochemical properties could be attributed to a combination of buffering, conducting, and confining effects of graphene. Besides pristine Fe3O4, partly substituted Fe3O4, such as ZnFe2O4,17 CoFe2O4 (ref. 18 and 19) and MnFe2O4,20 also exhibited obviously improved electrochemical performance when forming composites with graphene. In particular, the MnFe2O4/graphene composite showed better electrochemical performance than MnO–Fe2O3/graphene composite, where a mixture of MnO and Fe2O3 was used instead of MnFe2O4, which indicated the advantage of the mixed oxides.
Zinc ferric oxide (ZnFe2O4), which can be considered as an analogue of Fe3O4 by replacing Fe2+ with Zn2+, also exhibited high-performance Li-storage properties.21–24 ZnFe2O4/graphene composites, prepared by hydrothermal or solvothermal routes, have been used as photocatalyst,25 adsorbent,26 and anode of Li-ion batteries.27 In our previous work, we found that ZnFe2O4 can be uniformly anchored on graphene via an in situ solvothermal route and showed better rate capability and cycling stability.17 However, the primary ZnFe2O4 nanocrystals (around 5 nm) still tend to aggregate into large secondary particles (200 nm) even though in the presence of graphene. In this work, an optimized hydrothermal method was used to prepare ZnFe2O4/graphene hybrid. Deionized (DI) water instead of ethylene glycol (EG) was used as the medium for the synthetic reaction. We found that metallic ions can dissolve in water more easily than in EG and that graphite oxide (GO) can be dispersed by water more easily than by EG. Thus, the use of water as the medium makes it easier to disperse the metallic ions on GO sheets more easily by electrostatic force.28 KOH instead of urea was used as mineralizer to promote the hydrolysis of metallic ions. In addition, the reduction of GO can be promoted in the strong alkaline solution, for example NaOH or KOH.29 After rapid reduction of GO, the re-stacking of the hydrophobic graphene sheets can confine the ZnFe2O4 crystals effectively, preventing the further crystal growth. As a result, ultrafine ZnFe2O4 nanocrystals (below 10 nm) are well dispersed on large-sized graphene, resulting in the formation of a sheet-like structure. In addition, the reaction time can be decreased from 48 h to 24 h under this reaction condition. The resulting ZnFe2O4/graphene hybrid exhibits superior rate capability and high-rate cycling stability. In addition, the anode behavior of ZnFe2O4/graphene was also studied by constructing a ZnFe2O4/graphene–LiFePO4/C full cell, where nanosized, environmentally friendly LiFePO4/C was used as the cathode.
2. Experimental
2.1 Preparation of ZnFe2O4/G nanohybrids
GO (72 mg), prepared by a modified Hummer's method,30 was added into 50 mL of DI water with sonication to form a uniform solution. Then, 1 mmol of Zn(NO3)2·6H2O (Alfa Aesar) and 2 mmol of Fe(NO3)3·6H2O (Alfa Aesar) were added slowly to the above solution with stirring for 30 min. Afterwards, 2.24 g of KOH was added to the above mixed solution to reach a concentration of 0.5 mol L−1. After being stirred for another 2 h, the solution was transferred to a Teflon-lined stainless steel autoclave (100 mL in capacity) and heated in an electric oven at 200 °C for 24 h. The resulting product was collected by centrifugation, washed with DI water and absolute ethanol for several times and dried at 40 °C under vacuum for 8 h. The obtained product is named ZnFe2O4/G. Control experiments were also carried out to prepare bare ZnFe2O4 and bare graphene using the similar route without the addition of GO or Fe(NO3)3·6H2O/Zn(NO3)2·6H2O precursors. In the following sections, ZnFe2O4 is used to represent bare ZnFe2O4. A simple mixture of ZnFe2O4 and graphene was also prepared by mixing 12.4 wt% bare graphene and 87.6 wt% bare ZnFe2O4. The simple mixture is named ZnFe2O4/G-SM.2.2 Preparation of LiFePO4/C
LiFePO4 was prepared by a hydrothermal method using H3PO4, FeSO4·7H2O and LiOH·H2O as the precursors with a molar ratio of 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.7. The hydrothermal reaction was performed at 180 °C for 10 h. The resulting product was washed with DI water and absolute ethanol repeatedly and dried at 100 °C under vacuum for 8 h. LiFePO4/C was obtained by firing a homogeneous mixture of the as-prepared LiFePO4 and sucrose at 650 °C for 3 h.
2.7. The hydrothermal reaction was performed at 180 °C for 10 h. The resulting product was washed with DI water and absolute ethanol repeatedly and dried at 100 °C under vacuum for 8 h. LiFePO4/C was obtained by firing a homogeneous mixture of the as-prepared LiFePO4 and sucrose at 650 °C for 3 h.
2.3 Materials characterization
X-ray diffraction (XRD) patterns were collected on a Rigaku D/Max-2550pc powder diffractometer equipped with Cu Kα radiation (λ = 1.541E). X-ray photoelectron spectroscopy (XPS) was recorded on a KRATOS AXIS ULTRA-DLD spectrometer with a monochromatic Al Kα radiation (hν = 1486.6 eV). The microstructure of the products was analyzed by field emission scanning electron microscopy (SEM) on a FEI-sirion microscope, and transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) on a JEM 2100F microscope. Raman spectra were measured on a Jobin-Yvon Labor Raman HR-800 Raman system using a 514.5 nm Ar-ion laser at 10 mW. Thermogravimetric (TG) analysis of ZnFe2O4/G was conducted on a DSCQ1000 instrument from 22 to 800 °C at a ramp rate of 10 °C min−1 in air. The carbon content analysis in LiFePO4/C was conducted on a Flash EA 1112 tester.2.4 Electrochemical measurements
The electrode slurry was made by mixing 75 wt% active material (ZnFe2O4, ZnFe2O4/G, ZnFe2O4/G-SM, LiFePO4/C), 15 wt% acetylene black (AB) and 10 wt% polyvinylidene fluoride (PVDF) in N-methyl pyrrolidone (NMP) with magnetic stirring for 2 h. The slurry was spread onto Ni foam (for ZnFe2O4, ZnFe2O4/G and ZnFe2O4/G-SM) or Al foil (for LiFePO4/C) to fabricate the working electrodes. The electrodes were dried at 100 °C under vacuum for 8 h before the batteries assembly. CR2025 coin-type half cells were assembled in an argon-filled glove box using metallic Li foil as the counter electrode, 1 M LiPF6 in ethylene carbonate–dimethyl carbonate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) as the electrolyte, and Celgard 2300 membrane as the separator. Coin-type full cells were assembled in a similar manner by replacing Li foils with the LiFePO4/C electrodes. The cells were galvanostatically charged–discharged on a Neware battery tester (Shenzhen, China) at various current densities. The voltage range is 0.005–3.0 V (vs. Li/Li+) for half cells and 1.0–3.5 V (LiFePO4/C vs. ZnFe2O4/G, ZnFe2O4 or ZnFe2O4/G-SM) for full cells. Cyclic voltammetry (CV) measurements were conducted on an Arbin BT2000 system at a scan rate of 0.1 mV s−1 between 0.005 and 3.0 V. Electrochemical impedance spectroscopy (EIS) measurement was carried out on a CHI660C electrochemistry workstation by applying an AC signal of 5 mV amplitude over the frequency range of 105 to 10−2 Hz.
1 by volume) as the electrolyte, and Celgard 2300 membrane as the separator. Coin-type full cells were assembled in a similar manner by replacing Li foils with the LiFePO4/C electrodes. The cells were galvanostatically charged–discharged on a Neware battery tester (Shenzhen, China) at various current densities. The voltage range is 0.005–3.0 V (vs. Li/Li+) for half cells and 1.0–3.5 V (LiFePO4/C vs. ZnFe2O4/G, ZnFe2O4 or ZnFe2O4/G-SM) for full cells. Cyclic voltammetry (CV) measurements were conducted on an Arbin BT2000 system at a scan rate of 0.1 mV s−1 between 0.005 and 3.0 V. Electrochemical impedance spectroscopy (EIS) measurement was carried out on a CHI660C electrochemistry workstation by applying an AC signal of 5 mV amplitude over the frequency range of 105 to 10−2 Hz.
3. Results and discussion
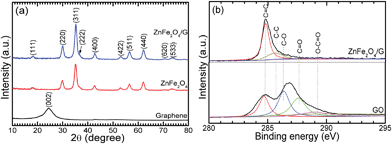
Fig. 1a shows the XRD patterns of ZnFe2O4/G, ZnFe2O4 and graphene. All the diffraction peaks of ZnFe2O4/G and ZnFe2O4 can be indexed to the spinel ZnFe2O4 phase (space group Fd3m, JCPDS card no. 82-1049). The graphene content is estimated to be 12.4 wt% according to the TG analysis (ESI, Fig. S1†). At a relatively high content, however, the diffraction peak of graphene is unobservable, while bare graphene clearly exhibits its (002) characteristic peak at around 2θ = 25°. The absence of the graphene peak is due to the inhibited restacking of the graphene sheets after reduction by attaching ZnFe2O4 nanocrystals as the spacers. Note that the ZnFe2O4 exhibits broad diffraction peaks, implying a small size of the particles.Fig. 1b gives the C1s XPS of ZnFe2O4/G and GO. The XPS can be fitted into five peaks, corresponding to carbon atoms in different forms: non-oxygenated carbon (C–C, 285.6 eV, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, 284.8 eV), carbon in C–O group (epoxy or hydroxyl, 286.3 eV), carbonyl carbon (C
C, 284.8 eV), carbon in C–O group (epoxy or hydroxyl, 286.3 eV), carbonyl carbon (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 287.6 eV) and carboxyl carbon (O–C
O, 287.6 eV) and carboxyl carbon (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 289.0 eV).31 Note that the peak intensity of C–O, C
O, 289.0 eV).31 Note that the peak intensity of C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–C
O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups demonstrates a remarkable decrease for ZnFe2O4/G after the hydrothermal reaction, indicating a sufficient reduction of GO. The formation of ZnFe2O4 is also confirmed by XPS survey spectra and Fe 2p and Zn 2p XPS spectra (ESI, Fig. S2†) and the reduction of GO is further examined by Raman spectra (ESI, Fig. S3†).
O groups demonstrates a remarkable decrease for ZnFe2O4/G after the hydrothermal reaction, indicating a sufficient reduction of GO. The formation of ZnFe2O4 is also confirmed by XPS survey spectra and Fe 2p and Zn 2p XPS spectra (ESI, Fig. S2†) and the reduction of GO is further examined by Raman spectra (ESI, Fig. S3†).
The morphology of ZnFe2O4/G was investigated by SEM, TEM and HRTEM as shown in Fig. 2. Fig. 2a shows a typical SEM image of the ZnFe2O4/G hybrid. Clearly, the hybrid exhibits a sheet-like structure constructed by ZnFe2O4 nanocrystals attached on graphene nanosheets. High-magnification SEM image in Fig. 2b indicates that the size of the ZnFe2O4 nanocrystals is rather small, in agreement with the XRD result. TEM image in Fig. 2c reveals that the graphene sheet is decorated with small ZnFe2O4 crystals with a size below 10 nm. Fig. 2d gives the lattice resolved HRTEM image of ZnFe2O4/G. The fringe spacings of 0.25 nm correspond to the interplanar distance of (311) planes of ZnFe2O4. HRTEM image also indicates that the graphene is in few-layer form (below 10 layers).
Based on the above analyses, a possible formation mechanism of the ZnFe2O4/G hybrid is proposed and is schematically illustrated in Fig. 3. It suggests that four factors determine the formation of this unique nanohybrid: (i) GO is sufficiently exfoliated into graphene oxide sheets in DI water by vigorous ultrasonic treatment; (ii) positively charged Zn2+/Fe3+ ions are uniformly dispersed onto the negatively charged graphene oxide sheets by electrostatic force; (iii) hydrolysis of Zn2+/Fe3+ ions and reduction of GO into graphene occur simultaneously during the hydrothermal reaction; (iv) crystal growth of ZnFe2O4 is inhibited because of the dispersing and confining effects of graphene. Otherwise, ZnFe2O4 nanocrystals tend to aggregate in the absence of graphene (ESI, Fig. S4†).
Fig. 4a shows the CV plots of ZnFe2O4/G scanned at 0.1 mV s−1 for the first three cycles. A large reduction peak at around 0.5 V can be observed during the first cathodic scan, corresponding to the irreversible conversion from ZnFe2O4 to LiZn/Fe/Li2O upon Li uptake.18 This peak is also partly overlapped with the peak related to formation of the solid electrolyte interface (SEI) layer. Note that during the second and third cathodic scans, the peaks are shifted to a higher potential and separated into two peaks at around 0.8 and 1.2 V, indicating that the reduction of Fe2O3 proceeds through a two-step process, while the corresponding anodic peaks are at 1.6 and 1.9 V. After the first scan, both the oxidation and reduction peaks are almost overlapped, suggesting reversible conversion reactions between ZnO/Fe2O3 and LiZn/Fe/Li2O lattice.18
Fig. 4b shows the charge (de-lithiation)-discharge (lithiation) curves of ZnFe2O4/G in the initial three cycles at 50 mA g−1. The capacity of ZnFe2O4/G is calculated based on the total mass of ZnFe2O4 and graphene. The first charge and discharge capacities of ZnFe2O4/G are 839 and 1361 mA h g−1, respectively, while those of bare ZnFe2O4 are 557 and 956 mA h g−1 (ESI, Fig. S5†). The theoretical capacity of ZnFe2O4/G is estimated to be 746 mA h g−1 according to the obtainable capacity of graphene32 and the theoretical capacity of ZnFe2O4 (1000 mA h g−1) according to the reaction (ZnFe2O4 ↔ Li0.5ZnFe2O4 ↔ Li2ZnFe2O4 → Li2O + Li–Zn + Fe).18 The extra capacity of ZnFe2O4/G is attributed possibly to the synergetic effect between superfine ZnFe2O4 nanocrystals and graphene. On one hand, graphene can uniformly disperse ZnFe2O4 nanocrystals, leading to a good electrolyte wetting and high utilization of the active particles. On the other hand, the presence of ZnFe2O4 nanocrystals makes it easier for graphene to expose to the electrolyte, especially for the edges and defects on graphene that offer additional Li-storage sites.33 For both ZnFe2O4 and ZnFe2O4/G, the first irreversible capacity can be attributed to the formation of the SEI layer as mentioned above.
The cycling stability of ZnFe2O4/G at high charge current densities was shown in Fig. 4c. The cells were charged at 400 and 800 mA g−1 with the discharged current density fixed at 50 mA g−1. It is evident that ZnFe2O4/G can exhibit an excellent long-term cycling stability at high current densities. At 400 mA g−1, ZnFe2O4/G can deliver a first charge capacity of 763 mA h g−1. After 300 cycles, the hybrid still maintains a high charge capacity of 549 mA h g−1, corresponding to capacity retention of 72%. Even at 800 mA g−1 (over 1 C), the hybrid can still deliver a first charge capacity of 701 mA h g−1. The charge capacity can be kept at 464 mA h g−1 after 300 cycles, higher than the theoretical capacity of graphite (372 mA h g−1), indicative of its excellent high-rate cycling stability. By contrast, bare ZnFe2O4 shows a rather poor cycling stability even though at a low current density of 50 mA g−1. After 100 cycles, the charge capacity of bare ZnFe2O4 drops rapidly to 101 mA h g−1. For ZnFe2O4/G prepared in EG in our previous work,17 its charge capacity decreased to 398 mA h g−1 after 90 cycles at 400 mA g−1 even though it has a much higher graphene content of 36.3 wt%. Ex situ TEM was also used to clarify the different electrochemical properties between the two ZnFe2O4/G composites. As seen in Fig. 5, both large ZnFe2O4 spheres and small ZnFe2O4 particles on graphene cannot be seen after cycling, indicating repeated electrochemical grinding of the particles or spheres accompanied by their pulverization upon large volume changes. Instead, sheet-like structure appears for both samples with the latter (Fig. 5c) exhibiting smaller size, more uniform size distribution and less significant particles aggregation. In addition, the latter is composed smaller particles with voids in between these particles (Fig. 5d) which can buffer the volume changes. As a result, the ZnFe2O4/G with small sized ZnFe2O4 exhibits better electrochemical properties than that with large sized ZnFe2O4.
Of note is that at high current density the charge capacity of ZnFe2O4/G decreases rapidly in the initial cycles, increases gradually during the subsequent cycling, and then shows a slight change with cycling. The phenomenon is not well understood yet but it maybe related to the progressive activation and structural rearrangement of the electrode with cycling (Fig. 5). For ZnFe2O4/G, despite the small size of ZnFe2O4 crystals and the buffering effect of graphene, volume changes also occur upon lithiation/de-lithiation, during which deactivation of some active particles is unavoidable due to the failure of physical contact between the particles, leading to rapid capacity fade in the initial cycles. These deactivated particles may be activated again provided that they are still anchoring on conductive graphene, which results in subsequent capacity rise. After that, the capacity generally has a tendency to decay. The fluctuation of the capacity during this period is likely due to pseudocapacitive character of the polymeric film34 that undergoes structural rearrangement during cycling. For bare ZnFe2O4, the active material will undergo a continuous exfoliation without the confining effect of graphene. To confirm this, a simple mixture of ZnFe2O4 and graphene (12.4 wt%, ZnFe2O4/G-SM) was also prepared. ZnFe2O4/G-SM shows continuous capacity decay because of the poor contact between the active material and graphene as seen Fig. 4e.
It is generally accepted that nanoengineering will bring an obvious improvement in electrochemical properties of TMOs as in the case of Fe3O4.35 However, for our bare ZnFe2O4 sample, its electrochemical properties are rather poor despite the nanostructure. Ex situ TEM in Fig. 5 has revealed that pulverization and electrochemical grinding of graphene-confined ZnFe2O4 particles occur regardless of the particle size. This means that without the confining effect of graphene, significant exfoliation of the active material will be unavoidable upon repeated cycling, which leads to rapid capacity fade. By contrast, the excellent cycling stability of Fe3O4 may be due to the stable structure of the Fe3O4 nanoparticles.35 Therefore, for the nanosized ZnFe2O4, a conductive support, for example graphene, is necessary to improve its electrochemical properties.
It is clear that graphene plays an important role in enhancing the electrochemical properties of ZnFe2O4 via the following aspects: (1) graphene can tightly immobilize the ZnO/Fe2O3 or LiZn/Fe/Li2O particles, guaranteeing their long-term electrochemical activity; (2) the high Li-ion and electronic conductivities of graphene make it possible for rapid electrochemical reactions, endowing the hybrid with high capacity at high current density; (3) the unique sheet-like hybrid structure offers sufficient buffering room for volume variation.
Fig. 4d compares the rate capability between ZnFe2O4/G and ZnFe2O4. The cells are charged at various current densities and discharge at 50 mA g−1. It is obvious that the rate capability of ZnFe2O4 can be significantly enhanced by loading it on graphene. At 1600 mA g−1, ZnFe2O4/G can still deliver a charge capacity of 340 mA h g−1, comparable with the theoretical value of graphite (372 mA h g−1). On the contrary, the charge capacity of bare ZnFe2O4 at such a high current density is only 86 mA h g−1. Even at 6400 mA g−1 (over 8 C), a charge capacity of 135 mA h g−1 is still obtainable for ZnFe2O4/G. Note that when the current density is shifted to 50 mA g−1, the capacity of ZnFe2O4/G can be recovered to 822 mA g−1, close to its initial value, implying that the microstructure of the ZnFe2O4/G is preserved after high-rate cycling. Again, the improved rate capability of ZnFe2O4/G is due to the presence of graphene. First, graphene offers 2D conductive channels for ZnO/Fe2O3 (or LiZn/Fe/Li2O) particles; second, the free space in the hybrid structure of ZnFe2O4/G facilitates easy wetting of the active materials by the electrolyte; third, the rather small size of ZnO/Fe2O3 (or LiZn/Fe/Li2O) particles makes it easy for rapid Li-ion transport both at the particle/electrolyte interface and in the bulk particle.
Fig. 6 shows the Nyquist plots of ZnFe2O4/G and ZnFe2O4 after 5 and 40 cycles. The Nyquist plots are composed of two partially overlapped semicircles at high and medium frequency regions and a slopping line at the low frequency region. The first semicircle is correlated to Li-ion transport resistance through the SEI layer, the second one corresponds to the charge transfer resistance, the slopping line is related to the Li-ion diffusion in the bulk material, and the intercept on Z′ axis at high frequency region is related to the electrolyte resistance. The Nyquist plots are fitted by the equivalent circuit shown in the inset Fig. 6. In the equivalent circuit, Re represents electrolyte resistance, Rf and Q1 denote SEI layer resistance and the dielectric relaxation capacitance, Rct and Q2 represent the charge transfer resistance and the double layer capacitance, and Zw is the bulk diffusion resistance. A constant phase element (CPE) instead of a capacitor (C) is used due to the dispersion effect. The CPE can be expressed as:36
YCPE = Ycωn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(nπ/2) + jYcωn cos(nπ/2) + jYcωn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(nπ/2) sin(nπ/2)
| (1) |
 | ||
| Fig. 6 Nyquist plots of ZnFe2O4/G and ZnFe2O4 after 5 and 40 cycles. The inset gives the equivalent circuit. | ||
| Samples | Re (Ω) | Rf (Ω) | Q1 | Rct (Ω) | Q2 | |||
|---|---|---|---|---|---|---|---|---|
| Y | n | Y | n | |||||
| ZnFe2O4/G | 5 cycles | 6.0 | 88.7 | 5.5 × 10−3 | 0.42 | 32.7 | 1.7 × 10−4 | 0.60 |
| 40 cycles | 8.4 | 102.7 | 2.8 × 10−3 | 0.45 | 47.0 | 1.1 × 10−4 | 0.62 | |
| ZnFe2O4 | 5 cycles | 4.3 | 90.6 | 2.0 × 10−3 | 0.58 | 100.9 | 5.3 × 10−5 | 0.70 |
| 40 cycles | 5.5 | 74.5 | 2.0 × 10−3 | 0.85 | 194.4 | 2.4 × 10−5 | 0.74 | |
A ZnFe2O4/G–LiFePO4/C full cell has been fabricated to investigate the anode behavior of ZnFe2O4/G in full cell. The LiFePO4/C composite was prepared by the hydrothermal route followed by carbon coating as described in the Experimental section. Phase purity of LiFePO4 and nanostructure of LiFePO4/C are confirmed by XRD (ESI, Fig. S7a†) and SEM (ESI, Fig. S7b†). The carbon content in LiFePO4/C is around 4 wt%. The capacity of the full cell is anode limited by adjusting weight ratio of LiFePO4/C to ZnFe2O4/G at around 14. The first charge (Li is from LiFePO4 to ZnFe2O4/G) curve is characteristic of a quasi-plateau at 1.5–2.5 V and a plateau at 2.5 V, yielding a high first charge capacity of around 2000 mA h g−1 (ESI, Fig. S8†). The first discharge (Li is from ZnFe2O4 to LiFePO4) capacity is 805 mA h g−1, close to the value in the half cell and much higher than that of graphite. The large first irreversible capacity is due to the formation of the SEI layer on ZnFe2O4/G similar to that in half cell. As seen in Fig. 7, after the first cycle, the charge and discharge are reversible with quasi-plateaus formed at around 2 V. Although the cycling stability is not satisfactory yet, it can be improved by optimizing the LiFePO4/C cathode and assembly technique of the full cells.
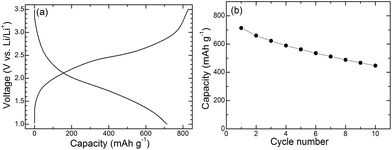 | ||
| Fig. 7 (a) The second charge and discharge curves and (b) cycling stability of the ZnFe2O4/G–LiFePO4/C full cell at 100 mA g−1. | ||
4. Conclusions
In summary, a ZnFe2O4/G nanohybrid has been successfully synthesized by a facile in situ route. The ZnFe2O4/G hybrid shows better cycling stability and rate capability than bare ZnFe2O4. At a charge current density of 800 mA g−1 (over 1 C), the hybrid can still deliver a first charge capacity of 701 mA h g−1 that can be maintained at 464 mA h g−1 after 300 cycles. The excellent high-rate cycling stability is attributed to the combined conducting, confining and dispersing effects of graphene. In addition, the unique 2D sheet-like nanostructure maximizes the exposure of the active material to the electrolyte and buffers the volume changes. In the anode limited ZnFe2O4/G–LiFePO4/C full cell, ZnFe2O4/G can yield a high initial discharge capacity of 805 mA h g−1 at 100 mA g−1. The excellent electrochemical properties of ZnFe2O4/G make it a promising anode material for Li-ion batteries.Acknowledgements
The authors appreciate the support from National Basic Research Program of China (2013CB934001), the National Natural Science Foundation of China (no. 51101139), the Ph.D. Programs Foundation of Ministry of Education of China (no. 20100101120024), the Qianjiang Talents Project of Science Technology Department of Zhejiang Province (2011R10021), and Key Science and Technology Innovation Team of Zhejiang Province under grant number 2010R50013.References
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- J. Cabana, L. monconduit, D. Larcher and M. R. Palacín, Adv. Mater., 2010, 22, E170–E192 CrossRef CAS PubMed.
- T. H. Kim, J. S. Park, S. K. Chang, S. Choi, J. H. Ryu and H. K. Song, Adv. Energy Mater., 2012, 2, 860–872 CrossRef CAS.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS PubMed.
- L. W. Ji, Z. Lin, M. Alcoutlabi and X. W. Zhang, Energy Environ. Sci., 2011, 4, 2682–2699 CAS.
- H. B. Wu, J. S. Chen, H. H. Hng and X. W. Lou, Nanoscale, 2012, 4, 2526–2542 RSC.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- M. D. Stoller, S. Park, Y. W. Zhu, J. H. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498–3502 CrossRef CAS PubMed.
- S. Park, J. H. An, I. W. Jung, R. D. Piner, S. J. An, X. S. Li, A. Velamakanni and R. S. Ruoff, Nano Lett., 2009, 9, 1593–1597 CrossRef CAS PubMed.
- C. Lee, X. D. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS PubMed.
- P. L. Taberna, S. Mitra, P. Poizot, P. Simon and J. M. Tarascon, Nat. Mater., 2006, 5, 567–573 CrossRef CAS PubMed.
- G. M. Zhou, D. W. Wang, F. Li, L. L. Zhang, N. Li, Z. S. Wu, L. Wei, G. Q. Lu and H. M. Cheng, Chem. Mater., 2010, 22, 5306–5313 CrossRef CAS.
- B. J. Li, H. Q. Cao, J. Shao, M. Z. Qu and J. H. Warner, J. Mater. Chem., 2011, 21, 5069–5075 RSC.
- L. W. Ji, Z. K. Tan, T. R. Kuykendall, S. Aloni, S. D. Xun, E. Lin, V. Battaglia and Y. G. Zhang, Phys. Chem. Chem. Phys., 2011, 13, 7170–7177 RSC.
- S. K. Behera, Chem. Commun., 2011, 47, 10371–10373 RSC.
- W. F. Chen, S. R. Li, C. H. Chen and L. F. Yan, Adv. Mater., 2011, 23, 5679–5683 CrossRef CAS PubMed.
- W. T. Song, J. Xie, S. Y. Liu, G. S. Cao, T. J. Zhu and X. B. Zhao, New J. Chem., 2012, 36, 2236–2241 RSC.
- S. Y. Liu, J. Xie, C. C. Fang, G. S. Cao, T. J. Zhu and X. B. Zhao, J. Mater. Chem., 2012, 22, 19738–19743 RSC.
- H. Xia, D. D. Zhua, Y. S. Fu and X. Wang, Electrochim. Acta, 2012, 83, 166–174 CrossRef CAS PubMed.
- Y. L. Xiao, J. T. Zai, L. Q. Tao, B. Li, Q. Y. Han, C. Yu and X. F. Qian, Phys. Chem. Chem. Phys., 2013, 15, 3939–3945 RSC.
- Y. Sharma, N. Sharma, G. V. Subba Rao and B. V. R. Chowdari, Electrochim. Acta, 2008, 53, 2380–2385 CrossRef CAS PubMed.
- X. W. Guo, X. Lu, X. P. Fang, Y. Mao, Z. X. Wang, L. Q. Chen, X. X. Xu, H. Yang and Y. N. Liu, Electrochem. Commun., 2010, 12, 847–850 CrossRef CAS PubMed.
- Y. Ding, Y. F. Yang and H. X. Shao, Electrochim. Acta, 2011, 56, 9433–9438 CrossRef CAS PubMed.
- Y. F. Deng, Q. M. Zhang, S. D. Tang, L. T. Zhang, S. N. Deng, Z. C. Shi and G. H. Chen, Chem. Commun., 2011, 47, 6828–6830 RSC.
- Y. S. Fu and X. Wang, Ind. Eng. Chem. Res., 2011, 50, 7210–7218 CrossRef CAS.
- P. Fei, M. Zhong, Z. Q. Lei and B. T. Su, Mater. Lett., 2013, 108, 72–74 CrossRef CAS PubMed.
- X. L. Chen, B. Cheng, H. Y. Xu, J. Yang and Y. T. Qian, Chem. Lett., 2012, 41, 639–641 CrossRef CAS.
- D. Li, M. B. Müller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS PubMed.
- X. B. Fan, W. C. Peng, Y. Li, X. Y. Li, S. L. Wang, G. L. Zhang and F. B. Zhang, Adv. Mater., 2008, 20, 4490–4493 CrossRef CAS.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339–1339 CrossRef CAS.
- H. J. Shin, K. K. Kim, A. Benayad, S. M. Yoon, H. K. Park, I. S. Jung, M. H. Jin, H. K. Jeong, J. M. Kim, J. Y. Choi and Y. H. Lee, Adv. Funct. Mater., 2009, 19, 1987–1992 CrossRef CAS.
- S. Y. Liu, J. Xie, Y. X. Zheng, G. S. Cao, T. J. Zhu and X. B. Zhao, Electrochim. Acta, 2012, 66, 271–278 CrossRef CAS PubMed.
- D. Y. Pan, S. Wang, B. Zhao, M. H. Wu, H. J. Zhang, Y. Wang and Z. Jiao, Chem. Mater., 2009, 21, 3136–3142 CrossRef CAS.
- S. Laruelle, S. Grugeon, P. Poizot, M. Dollé, L. Dupont and J. M. Tarascon, J. Electrochem. Soc., 2002, 149, A627–A634 CrossRef CAS PubMed.
- S. K. Behera, J. Power Sources, 2011, 196, 8669–8674 CrossRef CAS PubMed.
- T. Piao, S. M. Park, C. H. Doh and S. J. Moon, J. Electrochem. Soc., 1999, 146, 2794–2798 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: TG curve of ZnFe2O4/G, XPS survey, Zn2p and Fe2p XPS spectra, Raman spectra of ZnFe2O4/G, SEM image of bare ZnFe2O4, the first three charge and discharge curves of bare ZnFe2O4, SEM and TEM images of ZnFe2O4/G prepared in EG, XRD patterns and SEM image of LiFePO4/C, and the first charge and discharge curves of the ZnFe2O4/G–LiFePO4/C full cell. See DOI: 10.1039/c3ra46904b |
| This journal is © The Royal Society of Chemistry 2014 |