Remarkable decrease in binding strength for a phthalocyanine–fulleropyrrolidine non-covalent system in the presence of silver nanoparticles†
Anamika Ray and
Sumanta Bhattacharya*
The University of Burdwan, Department of Chemistry, Golapbag, Burdwan, West Bengal, India. E-mail: sum_9974@rediffmail.com
First published on 31st January 2014
Abstract
Silver nanoparticles with size of 5–15 nm reduce the binding constant of a phthalocyanine–fulleropyrrolidine system from ∼1.78 × 104 dm3 mol−1 to ∼1.31 × 103 dm3 mol−1 in toluene.
Phthalocyanine(Pc)–fullerene donor–acceptor conjugates have attracted immense attraction in the recent past due to their application in the construction of photosynthetic systems and photonic devices.1,2 Accordingly, both covalent and non-covalently connected Pc–fullerene dyads and triads have been designed and studied to understand the mechanistic details of the photoinduced electron transfer process relevant to the construction of artificial photosynthetic reaction centres.3–9 In a Pc–fullerene dyad, the role of the Pc is dual: first it is to function as an antenna and the second to act as a donor molecule once photo-excited. Moreover, the supramolecular methodology offers several advantages including ease of construction, ability to monitor through-bond electron transfer and flexible structures with the choice of adopted self-assembly protocols.10 An interesting aspect of the chemistry of fullerenes and Pc's is that they undergo spontaneous self-assembly phenomenon to each other, as a result of ground state complexation in solution.11–18 Our interest in this area of research has been to develop self-assembly protocols to form Pc–fullerene conjugates in presence of silver nanoparticles (AgNp) and to visualize the effect of intermolecular interactions on the physicochemical properties of these novel supramolecular systems. Metal nanoparticles have size dependent optical and electrical properties19 and in this respect, an important feature of the metal nanoparticles is the localized surface plasmon band resonance.20 Although there are some reports on interaction between fullerene and porphyrin in presence of silver and gold nanoparticles in recent past,21,22 there is no such investigations on non-covalent interaction between fullerene and Pc in presence of metal nanoparticles in solution. We anticipate that this sort of study is expected to provide information about the formation of supramolecular architecture which spans from the nanoscopic to the macroscopic level across multiple length scales, and affect the intermolecular binding strength between Pc and fullerene because of different wave-function mixing in the non-covalently linked donor–acceptor pair in presence of metal nanoparticles.
Both the Pc, namely, 2,3,9,10,16,17,23,24-octakis-(octyloxy)-29H,31H-phthalocyanine (1) and fullerene derivative, e.g., C60 pyrrolidine tris-acid ethyl ester (PyC60) as shown in Scheme 1, are obtained from Aldrich, USA and used after checking the purity of the compounds. The motivation behind selecting the PyC60 molecule as an electron acceptor comes from the work of Sessler et al. in which they have employed fulleropyrroline bearing a guanosine moiety as a recognition motif for the construction of Pc–C60 dyad system.23 It is observed that both PyC60 and 1 attract each other spontaneously in toluene and the binding constant (K) of 1–PyC60 system is determined to be 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 220 dm3 mol−1 as evidenced from UV-vis investigations (Fig. S1 in ESI†). The K value of 1–PyC60 system gets further support from steady state fluorescence measurements; K of 1–PyC60 system (see Table 1) is evaluated employing Benesi–Hildebrand equation.24 An excellent straight line plot is obtained with correlation factor of 0.99 (Fig. S2 in ESI†). Noteworthy is that, free-base Pc, a structural isomer of 1, is reported to exhibit ∼2.7 times higher magnitude of K compared to the average value of K, i.e., Kav, of 1–PyC60 system as reported in Table 1.14
220 dm3 mol−1 as evidenced from UV-vis investigations (Fig. S1 in ESI†). The K value of 1–PyC60 system gets further support from steady state fluorescence measurements; K of 1–PyC60 system (see Table 1) is evaluated employing Benesi–Hildebrand equation.24 An excellent straight line plot is obtained with correlation factor of 0.99 (Fig. S2 in ESI†). Noteworthy is that, free-base Pc, a structural isomer of 1, is reported to exhibit ∼2.7 times higher magnitude of K compared to the average value of K, i.e., Kav, of 1–PyC60 system as reported in Table 1.14
| System | K, dm3 mol−1 | Kav, dm3 mol−1 | Selectivity in K in presence of AgNp | |
|---|---|---|---|---|
| UV-vis | Fluorescence | |||
| 1–PyC60 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 320 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420 420 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 870 870 |
∼13.5 |
| 1–PyC60–AgNp | 940 | 1690 | 1315 | |
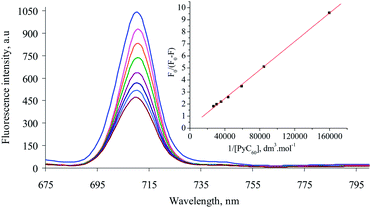
Absorption spectrophotometric investigations evoke a new physicochemical phenomenon when AgNp solution is added to 1 in toluene. It is observed that the Q absorption bands of 1 decrease systematically with increasing time while the absorbance value observed at 685 nm follow the opposite trend (Fig. 1). Very good isosbestic points are observed at 670 and 695 nm. The rate of dispersion of AgNp over the surface of 1 may be fitted nicely according to 1st order chemical kinetics mechanism (inset of Fig. 1) and the average value of k is estimated to be 0.0015 min−1. Dispersion of the nanoparticles over the surface of 1 is proposed to be complete after 4 h. Therefore, allowing this time, we have monitored the intermolecular interaction between 1 and PyC60 in presence of AgNp. Absorption spectrophotometric titration measurement reveals a remarkable decrease in the value of K for 1–PyC60 system in presence of AgNp by magnitude of ∼14.0 (Table 1). The UV-vis spectra and the modified BH plot25 are depicted in Fig. S3 (in ESI†). UV-vis investigations, therefore, may be treated as fingerprint observation in favour of inhibition in binding between 1 and PyC60 in presence of AgNp. Further support in this regard comes from steady state fluorescence investigations. Quenching in the intensity of fluorescence of 1 at emission band of 710 nm occurs efficiently in presence of PyC60 and AgNp (Fig. 2); like UV-vis investigations, a sharp decrease in the vale of K is observed (Table 1). An excellent liner correlation is obtained as evidenced from fluorescence BH plot24 (inset of Fig. 2). Table 1 envisages that average selectivity in binding in absence and presence of AgNp is estimated to be ∼13.5 which is supposed to be very much phenomenal incident in the supramolecular chemistry consisting any functionalized fullerene and Pc macrocycle.
 | ||
| Fig. 1 UV-vis spectral variation of 1 (4.75 × 10−6 mol dm−3) in presence of AgNp recorded in toluene at 298 K. | ||
To gain further insight into the intermolecular interaction between 1 and PyC60 in presence of AgNp, we have performed particle size analysis through dynamic light scattering (DLS) measurements. The size of AgNp is determined to be 4.18 nm (Fig. S4(a) in ESI†) as we selected AgNp having size of 5–15 nm (Aldrich, USA, catalogue no. 667838). When AgNp solution is added to 1, its size increased to 68.06 nm (Fig. S4(b) in ESI†). The large difference in these sizes before and after mixing with 1, i.e., 63.88 nm, corresponds to the thickness of the AgNp over the surface of 1. Furthermore, the DLS measurements find that the particle size of AgNp in 1–PyC60–AgNp composite mixture becomes 147.5 nm (Fig. 3) providing strong support in favour of the large decrease in the value of K for 1–PyC60 system. This may be the first example to track the occurrence of complexation by particle size analysis in presence of nanoparticles and, if so, of the size and extent of binding of the complex.
SEM measurements of AgNp in presence of 1 (Fig. S5(a) in ESI†) reveal formation of uneven sized particles ranging in smaller dimension, viz. 5 to 20 nm. However, the SEM image of composite mixture (i.e., 1 + PyC60 + AgNp) obtained from drop-casted films of the clusters on a stab made of copper indicates formation of well-defined packed cluster; formidable extent of increase in the particle size, i.e., 140–150 nm, is observed along the long axis (Fig. S5(b) in ESI†). The above results clearly demonstrate that the shape and the size of the surface holes on the 1–PyC60–AgNp nanostructure play an important role in controlling the formation of molecular cluster.
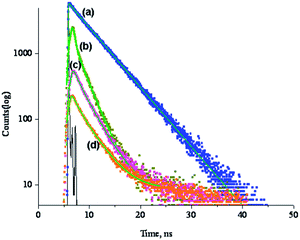
Apart from steady state fluorescence measurements, we have performed detailed nanosecond time-resolved fluorescence experiment for the investigated supramolecule in absence and presence of silver nanoparticles. The experiment has been carried out at a fixed concentration of 1. The time-resolved fluorescence measurement shows mono-exponential decay for uncomplexed 1; lifetime value of the singlet excited state (τs) of 1 is measured to be 6.175 ns (Fig. 4(a)). However, in presence AgNp, significant amount of quenching in the τs value of 1 (4.17 ns) takes place (Table 2). Moreover, in presence of AgNp, 1 decays according to bi-exponential fit and this is indicative of photochemical reaction at excited state (Fig. 4(b)). This phenomenon is very much consistent with the observation of chemical kinetics between 1 and AgNp in UV-vis investigations. Complexation of 1 with PyC60, in absence and presence of AgNp, reduces the τs value of 1 to ∼3 (Fig. 4(c)) and ∼3.3 times (Fig. 4(d)), respectively, compared to its original value reported earlier (Table 2). Utilizing the value of τs, rate constant of charge-separation of the excited singlet (ksCS) and quantum yield of charge-separation for the excited singlet (ΦsCS)25 have been enumerated and listed in Table 2. The notable feature is that, 1–PyC60 complex exhibits facile charge-separation in presence of AgNp as evidenced from large value of ΦsCS, i.e., 0.70. The lifetime experiment, therefore, suggests enhancement in the electrostatic interaction between Pc and fullerene in presence of AgNp.
| System | τ, ns | ksCS, sec−1 | ΦsCS |
|---|---|---|---|
| 1 | 6.17 | — | — |
| 1–AgNp | 4.17 | 7.76 × 107 | 0.35 |
| 1–PyC60 | 2.02 | 3.33 × 108 | 0.67 |
| 1–PyC60–AgNp | 1.86 | 3.75 × 108 | 0.70 |
In fluorescence microscope experiments, after exposure of 421 millisecond, 1 shows intense blue fluorescence (Fig. 5(a)). Following the addition of AgNp, fluorescence intensity of 1 is reduced significantly and much larger exposure time (1.6 second) is needed to get reliable image (Fig. 5(b)). PyC60 also reduces the fluorescence intensity of 1 (Fig. 5(c)) after complexation with 1. However, extraordinary feature is observed in case of 1–PyC60–AgNp ensemble when it exhibits intense blue fluorescence (Fig. 5(d)). It should be mentioned at this point that the bright blue colour is due to Surface Plasmon Resonance of AgNp that is peaked at a 450 nm wavelength as suggested by one of the learned reviewer.
In conclusion, AgNp inhibits the binding strength of Pc–PyC60 complex in solution. At the same time, considerable amount of enhancement in electrostatic interaction associated with charge-separation between Pc and PyC60 occurs in presence of silver nanoparticles.
We are thankful to Prof. S. C. Bhattacharya, JU, India for his helpful co-operations in DLS measurements; Prof. P. K. Ghosh and Dr S. Chakraborty, USIC, BU, India for fluorescence microscope and SEM measurements, respectively. AR thanks CSIR, New Delhi for providing Extended Senior Research Fellowship to her. Grant-In-Aid through Research Project of Sanction no. 01(2711)/13/EMR-II is also greatly acknowledged.
Notes and references
- G. Bottari, G. de la Torre, D. M. Guldi and T. Torres, Chem. Rev., 2010, 110, 6768 CrossRef CAS PubMed.
- M. E. El-Khouly, O. Ito, P. M. Smith and F. D'Souza, J. Photochem. Photobiol., C, 2004, 5, 79 CrossRef CAS PubMed.
- D. M. Guldi, B. M. Illescas, C. M. Atienza, M. Wielopolski and N. Martin, Chem. Soc. Rev., 2009, 38, 1587 RSC.
- G. Bottari, J. A. Suanzes, O. Trukhina and T. Torres, J. Phys. Chem. Lett., 2011, 2, 905 CrossRef CAS.
- A. de la Escosura, M. V. Martinez-Diaz, D. M. Guldi and T. Torres, J. Am. Chem. Soc., 2006, 128, 4112 CrossRef CAS PubMed.
- O. Ito and F. D'Souza, Molecules, 2012, 17, 5816 CrossRef CAS PubMed.
- O. Ito and F. D'Souza, Chem. Commun., 2009, 4913 Search PubMed.
- M. S. R. Morgade, M. E. P. Brzezinska, A. J. Athans, E. Carbonell, G. D. Miguel, D. M. Guldi, L. Echegoyen and T. Torres, J. Am. Chem. Soc., 2009, 131, 10484 CrossRef PubMed.
- A. Gouloumis, A. de la Escosura, P. Vázquez, T. Torres, A. Kahnt, D. M. Guldi, H. Neugebauer, C. Winder, M. Drees and N. S. Sariciftci, Org. Lett., 2006, 8, 5187 CrossRef CAS PubMed.
- B. Grimm, J. Schornbaum, H. Jasch, O. Trukhina, F. Wessendorf, A. Hirsch, T. Torres and D. M. Guldi, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 15565 CrossRef CAS PubMed.
- R. F. Enes, J.-J. Cid, O. Trukhina, A. Hausmann, A. Gouloumis, P. Vázquez, J. A. S. Cavaleiro, A. C. Tomé, D. M. Guldi and T. Torres, Chem. – Eur. J., 2012, 18, 1727 CrossRef CAS PubMed.
- M. Ince, M. V. Martinez-Diaz, J. Barbera and T. Torres, J. Mater. Chem., 2011, 21, 1531 RSC.
- C. B. KC, K. Ohkubo, P. A. Karr, S. Fukuzumi and F. D'Souza, Chem. Commun., 2013, 49, 7614 RSC.
- A. Ray, D. Goswami, S. Chattopadhyay and S. Bhattacharya, J. Phys. Chem. A, 2008, 112, 11627 CrossRef CAS PubMed.
- A. Ray, K. Santhosh, S. Chattopadhyay, A. Samanta and S. Bhattacharya, J. Phys. Chem. A, 2010, 114, 5544 CrossRef CAS PubMed.
- A. Ray, K. Santhosh and S. Bhattacharya, J. Phys. Chem. A, 2011, 115, 9929 CrossRef CAS PubMed.
- A. Ray, K. Santhosh and S. Bhattacharya, J. Phys. Chem. B, 2012, 116, 11979 CrossRef CAS PubMed.
- A. Ray, H. Pal and S. Bhattacharya, Spectrochim. Acta, Part A, 2014, 117, 686 CrossRef CAS PubMed.
- M. Pelton, J. Aizpurua and G. Bryant, Laser Photonics Rev., 2008, 2, 136 CrossRef CAS.
- P. K. Jain, X. Huang, I. H. El-Sayed and M. A. El-Sayed, Acc. Chem. Res., 2008, 41, 1578 CrossRef CAS PubMed.
- A. Kotiaho, R. M. Lahtinen, H.-K. Latvala, A. Efimov, N. V. Tkachenko and H. Lemmetyinen, Chem. Phys. Lett., 2009, 471, 269 CrossRef CAS PubMed.
- R. Mitra, S. Bhattacharya, A. K. Bauri and D. C. Mukherjee, Nanotechnol. Nanosci., 2012, 3, 46 Search PubMed.
- J. L. Sessler, J. Jayawickramarajah, A. Gouloumis, G. D. Pantos, T. Torres and D. M. Guldi, Tetrahedron, 2006, 62, 2123 CrossRef CAS PubMed.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703 CrossRef CAS.
- F. D'Souza, R. Chitta, S. Gadde, M. E. Zandler, A. L. McCarty, A. S. D. Sandanayaka, Y. Araki and O. Ito, Chem. – Eur. J., 2005, 11, 4416 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra46836d |
| This journal is © The Royal Society of Chemistry 2014 |