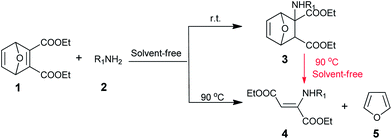
A facile synthesis of β-amino carbonyl compounds through an aza-Michael addition reaction under solvent-free conditions†
Chao Huang*ab,
Yanqing Yina,
Jiahui Guoa,
Jiong Wangab,
Baomin Fanab and
Lijuan Yang*ab
aKey Laboratory of Chemistry in Ethnic Medicinal Resources, State Ethnic Affairs Commission & Ministry of Education, Yunnan University of Nationalities, Kunming, 650500, China. E-mail: huang.chao@hotmail.com
bEngineering Research Center of Biopolymer Functional Materials of Yunnan, Yunnan University of Nationalities, Kunming, 650500, China
First published on 28th January 2014
Abstract
An efficient and eco-friendly process for the synthesis of β-amino carbonyl compounds was introduced in this paper. The oxanorbornene β-amino esters and β-enamine esters were successfully prepared from oxabornene and amines by using solvent-free aza-Michael addition reaction in the absence of any catalyst. Oxanorbornene β-amino esters were the major product at room temperature, but higher temperature (e.g. 90 °C) led to the formation of β-enamine esters. In addition, all of the target compounds were characterized by IR, 1H NMR, 13C NMR, and HR-MS. A possible reaction pathway was also proposed.
Introduction
β-Amino carbonyl structural units exist extensively in natural products and bioactive compounds.1 They are usually used as intermediates for the preparation of amino alcohols, diamines, β-amino acid derivatives and other nitrogen-containing molecules.2 As a result, their synthesis and application have received a great deal of recent attention in the fields of organic chemistry and medicinal chemistry.3The Mannich-type reaction could be a useful and practical protocol for constructing β-amino carbonyl units.4 Unfortunately, harsh reaction conditions and longer reaction time are commonly required.5 On the other hand, aza-Michael addition reaction, addition of an amine to an electron deficient alkene, offers an easy access to β-amino carbonyl compounds.6,7 To date, a number of synthetic approaches to β-amino carbonyl compounds have been established through aza-Michael addition.8 A variety of catalysts, such as acetic acid, boric acid,9 ionic liquid,10 cyclodextrin, transition metal,11 lanthanide halides, triflates or silica gel, solid salts and quaternary ammonium salt, have been developed for this purpose.12 However, drawbacks including use of expensive reagents or organic solvents, or an excess of catalyst and high temperature still exist.13 Thus, the development of an efficient and environmentally benign protocol could be highly desirable for the synthesis of β-amino carbonyl compounds.
Herein, an efficient and eco-friendly procedure for the synthesis of β-amino carbonyl compounds from oxabornene and amine via aza-Michael addition as depicted in Scheme 1 was disclosed without use of any catalyst and solvent. Oxanorbornene β-amino esters 3 and (Z)-β-enamine esters 4 were successfully prepared at r.t. and 90 °C, respectively.
Results and discussion
To establish the synthetic process for β-amino carbonyl compounds from oxabornene 1 and amines 2 through solvent-free aza-Michael addition reaction, the reaction of oxabornene 1 and 1-phenylpiperazine 2a were initially selected as a model reaction. As a result, diethyl 2-(4-phenylpiperazin-1-yl)-7-oxabicyclo [2.2.1]hept-5-ene-2,3-dicarboxylate 3a was obtained as the product. As shown in Table 1, acetone, THF, DCE and toluene as the solvent, the reaction gave poor results (Table 1, entries 1–4). To be delighted, the reaction ran well at r.t. without solvent under other identical conditions, leading to 75% isolated yield of 3a (entry 5). Accordingly, the solvent-free aza-Michael addition reaction was chosen for the further investigation.| Entry | Solvent | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a |
Temp. (°C) | Time | Yielda (%) |
|---|---|---|---|---|---|
| a Isolated yield by neutral alumina column chromatography. | |||||
| 1 | Acetone | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
r.t. | 12 h | 60 |
| 2 | THF | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
r.t. | 12 h | 59 |
| 3 | DCE | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
r.t. | 12 h | 64 |
| 4 | Toluene | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
r.t. | 12 h | 62 |
| 5 | Solvent-free | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
r.t. | 30 min | 75 |
| 6 | Solvent-free | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
30 | 30 min | 70 |
| 7 | Solvent-free | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
50 | 30 min | 63 |
| 8 | Solvent-free | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
70 | 30 min | 48 |
| 9 | Solvent-free | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
r.t. | 1 min | 53 |
| 10 | Solvent-free | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
r.t. | 1 min | 51 |
| 11 | Solvent-free | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
r.t. | 1 min | 75 |
| 12 | Solvent-free | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
r.t. | 1 min | 83 |
| 13 | Solvent-free | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
r.t. | 1 min | 68 |
The temperature has detrimental effect on the formation of oxanorbornene β-amino ester 3a. The yield decreased from 75% to 48% as the temperature went from r.t. up to 70 °C (Table 1, entries 5–8), indicating oxanorbornene β-amino ester may have poor stability at higher temperature. Interestingly, 1 min was enough to get better 3a yield (entry 11 vs. 5); on the other hand, excellent yield up to 83% was reached at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio of 1 to 2a (entries 9–13).
2 molar ratio of 1 to 2a (entries 9–13).
The applicability of various amines on solvent-free aza-Michael addition reaction was further investigated. The results showed that it is a general method to produce oxanorbornene β-amino esters 3 as listed in Table 2. Aliphatic amines have higher activity than aromatic counterparts (Table 2, entries 1–7 vs. 8–10), especially cyclic amines, ran much faster (entries 1–4), being in well agreement with the literature results.14 Whereas, longer reaction time was needed for aromatic amines for completing the reaction (entries 8–10), probably due to the electronic and steric effect. The yield of oxanorbornene β-amino esters 3 was also affected by the basicity and steric factor of the amino group and the nature of the carbonyl group (entries 3, 5 and 8).
| Entry | Amine 2 | Product 3 | Time | Isolated yielda (%) |
|---|---|---|---|---|
a The molar ratio of oxabornene 1 to amines 2 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; isolated yield by neutral alumina column chromatography; the progress of the reaction was monitored by TLC. 2; isolated yield by neutral alumina column chromatography; the progress of the reaction was monitored by TLC. |
||||
| 1 |  |
 |
1 min | 83 |
| 2 |  |
 |
1 min | 86 |
| 3 |  |
 |
1 min | 89 |
| 4 |  |
 |
1 min | 97 |
| 5 |  |
 |
0.5 h | 76 |
| 6 |  |
 |
1 h | 54 |
| 7 |  |
 |
5 h | 93 |
| 8 |  |
 |
12 h | 62 |
| 9 |  |
 |
10 h | 78 |
| 10 |  |
— | 24 h | Trace |
Unexpectedly, (Z)-β-enamine esters 4 and furan 5 can be obtained during the solvent-free aza-Michael addition reaction. When aliphatic amines as the substrates, e.g. 2b and 2e, just a trace amount of β-enamine ester 4 was formed and oxanorbornene β-amino ester 3 became the major product (Table 3, entries 1 and 2). In the case of aromatic amines, the yield of product 4 was slightly increased (entries 3 and 4). Those results encouraged us to further explore the reaction in order to develop novel access to β-amino carbonyl compound 4.
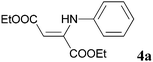
To establish the synthetic process for (Z)-β-enamine esters 4, a variety of reaction parameters and the results are summarized in Table 4. THF, toluene, acetone and DMF as the solvent, yields of diethyl 2-(phenylamino)fumarate 4a were found to be in the range of 27% to 63% at 90 °C for 24 h (Table 4, entries 1–4). Surprisingly, better yield was achieved for 6 h under solvent-less conditions (entries 5 vs. 1–4), suggesting solvent-free was favourable for the reaction. In addition, higher temperature is beneficial for the generation of β-amino ester 4a. The yield of 4a reached to 77% at 90 °C (entries 5). Further increasing the reaction temperature to 110 °C had negative effect on 4a yield (entries 10). And excellent yield was achieved at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio of 1 to 2a (entries 5 vs. 11–14).
2 molar ratio of 1 to 2a (entries 5 vs. 11–14).
| Entry | Solvent | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a |
Temp. (°C) | Time | Yielda/% |
|---|---|---|---|---|---|
| a Isolated yield by alumina column chromatography; the progress of the reaction was monitored by TLC. | |||||
| 1 | THF | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
90 | 24 h | 63 |
| 2 | Toluene | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
90 | 24 h | 41 |
| 3 | Acetone | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
90 | 24 h | 27 |
| 4 | DMF | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
90 | 24 h | 27 |
| 5 | Solvent-free | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
90 | 6 h | 77 |
| 6 | Solvent-free | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
r.t. | 5 d | 62 |
| 7 | Solvent-free | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
30 | 4 d | 65 |
| 8 | Solvent-free | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
50 | 3 d | 70 |
| 9 | Solvent-free | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
70 | 17 h | 72 |
| 10 | Solvent-free | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
110 | 6 h | 65 |
| 11 | Solvent-free | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 | 38 h | 13 |
| 12 | Solvent-free | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 | 36 h | 44 |
| 13 | Solvent-free | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
90 | 19 h | 59 |
| 14 | Solvent-free | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
90 | 5 h | 72 |
The generality of the reaction was examined and the results are presented in Table 5. A wide range of amines 2 reacted with oxabornene 1 to afford (Z)-β-enamine esters 4a–f. The reactions with alkylamine were easier to perform (Table 4, entries 3–6) in 0.5 h or 1 min. But aromatic amines needed longer time to react, as the weaker activity (entries 1 and 2 vs. 3–6). Generally, cyclic amines can produce enamines faster than open-chain amines (entries 4–6 vs. 3).
| Entry | Amine 2 | Product 4 | Time | Isolated yielda (%) | |
|---|---|---|---|---|---|
a The molar ratio of oxabornene 1 to amines 2 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; isolated yield by neutral alumina column chromatography; the progress of the reaction was monitored by TLC. 2; isolated yield by neutral alumina column chromatography; the progress of the reaction was monitored by TLC. |
|||||
| 1 | PhNH2 | 4a |  |
6 h | 77 |
| 2 | p-MePhNH2 | 4b |  |
4 h | 54 |
| 3 |  |
4c |  |
0.5 h | 42 |
| 4 |  |
4d |  |
1 min | 70 |
| 5 |  |
4e |  |
1 min | 61 |
| 6 |  |
4f |  |
1 min | 73 |
The plausible mechanism for the formation of oxanorbornene β-amino esters 3 and (Z)-β-enamine esters 4 was also proposed on the basis of the experimental results. As showed in Scheme 2, the reaction of the amine and oxabornene 1 generate the oxanorbornene β-amino ester 3 through aza-Michael addition reaction. Subsequently, 3 goes at higher temperature through retro-Diels–Alder reaction to produce (Z)-β-enamine esters 4. This is understandable that the thermodynamic product 4 forms at higher temperature; while oxanorbornene β-amino ester 3 is a major product at room temperature.15 Indeed, compound 4a and 5 were obtained from thermal degradation of 3h at 90 °C, identified by spectroscopy (see, ESI†).
Experimental
General method
All compounds were fully characterized by spectroscopic techniques. The NMR spectra were recorded on a Bruker-Avance 400 MHz spectrometer (1H: 400 MHz, 13C: 100 MHz) with tetramethylsilane (TMS) as the internal standard (δ 0.0 ppm), chemical shifts (δ) are expressed in ppm, and J values are given in Hz. Deuterated CDCl3 was used as a solvent. IR spectra were recorded on a FT-IR Thermo Nicolet Avatar 360 using a KBr pellet. The reactions were monitored by thin layer chromatography (TLC) using neutral alumina. The melting points were determined on an XT-4A melting point apparatus and are uncorrected. HRMS was performed on an Agilent LC-MSD TOF instrument.All chemicals and solvents were used as received without further purification unless otherwise stated. Column chromatography was performed on neutral alumina.
Preparation of diethyl 7-oxabicyclo[2.2.1]hepta-2,5-diene-2,3-dicarboxylate 1
Diethyl acetylenedicarboxylate 12 mmol and furan 60 mmol were placed in a sealed tube, which was heated at 100 °C for 20 hours. The reaction mixture was distilled under vacuum. The endoxide was obtained as a light yellow oil.16General procedure for the synthesis of oxanorbornene β-amino esters 3 through solvent-free aza-Michael addition reaction
A Schlenk was charged with 1 (0.4 mmol, 95.3 mg), amine 2 (0.8 mmol), and the solution was stirred for 1 minute to 6 days at room temperature until the 1 was completely consumed. The mixture was purified by flash column chromatography. The desired compounds (3a–j) were formed from 1 in yields: 54–97%.The data of the oxanorbornene β-amino esters 3
General procedure for the synthesis of (Z)-β-enamine esters 4
A Schlenk was charged with diethyl 7-oxabicyclo[2.2.1]hepta-2,5-diene-2,3-dicarboxylate 1 (0.4 mmol, 95.3 mg), amine 2 (0.8 mmol), and the solution was stirred for 1 minute to 6 days at 90 °C until 1 was completely consumed. The mixture was purified by flash column chromatography. The desired compounds 4 were formed from 1 in yields 42–77%.The data of the (Z)-β-enamine esters 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 931
931![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 471; found, 293.1477.
471; found, 293.1477.Conclusions
In summary, an efficient and eco-friendly protocol for the synthesis of β-amino carbonyl compounds through solvent-free aza-Michael addition reaction was disclosed. Meanwhile, two kinds of β-amino carbonyl compounds were obtained by tuning the reaction temperature. We believe these results stimulate further research efforts to develop β-amino carbonyl compounds and extend solvent-free aza-Michael addition reaction to pharmaceutical synthesis.Acknowledgements
The work was supported by the National Natural Science Foundation of China (Nos. 21202142, 21162042), the Key Project of Chinese Ministry of Education (No. 212161) and the Opening Foundation of Key Laboratory of Chemistry in Ethnic-medicine Resources (Yunnan Nationalities University), Ministry of Education (MZY1115).References
- (a) M. Misra, R. Luthra, K. L. Singh and K. Sushil, in Comprehensive Natural Products Chemistry, ed. D. H. R. Barton, K. Nakanishi and O. Meth-Cohn, Pergamon, Oxford, UK, 1999, vol. 4, p. 25 Search PubMed; (b) E. Busto, V. Gotor-Fernández and V. Gotor, Chem. Rev., 2011, 111, 3998 CrossRef CAS PubMed.
- (a) M. Liu and M. P. Sibi, Tetrahedron, 2002, 58, 7991 CrossRef CAS; (b) E. F. Kleinmann, Comprehensive Organic Synthesis, Pergamon, New York, NY, 1991 Search PubMed; (c) G. I. Georg, The Organic Chemistry of β-Lactams, VCH, New York, 1993 Search PubMed; (d) E. J. Corey and G. A. Reichard, Tertrahedron Lett., 1989, 30, 5207 CrossRef CAS; (e) E. A. Porter, X. Wang, H. S. Lee, B. Weisblum and S. H. Gellman, Nature, 2000, 404, 565 CrossRef CAS PubMed.
- (a) D. Sharma, Bandna, C. B. Reddy, S. Kumar, A. K. Shil, N. R. Guha and P. Das, RSC Adv., 2013, 3, 10335 RSC; (b) L. Yang, L. W. Xu, W. Zhou, L. Li and C. G. Xia, Tetrahedron Lett., 2006, 47, 7723 CrossRef CAS; (c) N. Morimotoa, Y. Takeuchi and Y. Nishinab, J. Mol. Catal. A: Chem., 2013, 368–369, 31 CrossRef; (d) A. G. Ying, L. Liu, G. F. Wu, G. Chen, X. Z. Chen and W. D. Ye, Tetrahedron Lett., 2009, 50, 1653 CrossRef CAS; (e) G. Imanzadeh, F. Kazemi, M. Zamanloo and Y. Mansoori, Ultrason. Sonochem., 2013, 20, 722 CrossRef CAS PubMed; (f) J. M. Yang, S. J. Ji, D. G. Gu, Z. L. Shen and S. Y. Wang, J. Organomet. Chem., 2005, 690, 2989 CrossRef CAS.
- (a) Y. Luan and S. E. Schaus, Org. Lett., 2011, 13, 2510 CrossRef CAS PubMed; (b) T. Ollevier and E. Nadeau, J. Org. Chem., 2004, 69, 9292 CrossRef CAS PubMed; (c) N. Azizi, L. Torkiyan and M. R. Saidi, Org. Lett., 2006, 8, 2079 CrossRef CAS PubMed; (d) B. Das, P. Balasubramanyam, B. Veeranjaneyulu and G. C. Reddy, Org. Chem., 2009, 74, 9505 CrossRef CAS PubMed; (e) M. Arend, B. Westermann and N. Risch, Angew. Chem., Int. Ed., 1998, 37, 1044 CrossRef; (f) S. Kobayashi and H. Ishitani, Chem. Rev., 1999, 99, 1069 CrossRef CAS PubMed.
- (a) Y. Hayashi, W. Tsuboi, I. Ashimine, T. Urushima, M. Shoji and K. Sakai, Angew. Chem., Int. Ed., 2003, 42, 3677 CrossRef CAS PubMed; (b) M. Arend, B. Westermann and N. Risch, Angew. Chem., Int. Ed., 1998, 37, 1044 CrossRef; (c) S. Kobayashi and H. Ishitani, Chem. Rev., 1999, 99, 1069 CrossRef CAS PubMed; (d) M. Arend, B. Westermann and N. Risch, Angew. Chem., Int. Ed., 1998, 37, 1044 CrossRef; (e) N. S. Josephsohn, M. L. Snapper and A. H. Hoveyda, J. Am. Chem. Soc., 2004, 126, 3734 CrossRef CAS PubMed; (f) A. J. A. Cobb, D. M. Shaw, D. A. Longbottom, J. B. Gold and S. V. Ley, Org. Biomol. Chem., 2005, 3, 84 RSC.
- (a) T. Meiresonne, S. Mangelinckx§ and N. D. Kimpe, Org. Biomol. Chem., 2011, 9, 7085 RSC; (b) X. J. Tang, Z. L. Yan, W. L. Chen, Y. R. Gao, S. Mao, Y. L. Zhang and Y. Q. Wang, Tetrahedron Lett., 2013, 54, 2669 CrossRef CAS.
- (a) D. L. Priebbenow, S. G. Stewart and F. M. Pfeffer, Tetrahedron Lett., 2012, 53, 1468 CrossRef CAS; (b) P. R. Krishna, A. Sreeshailam and R. Srinivas, Tetrahedron, 2009, 65, 9657 CrossRef CAS.
- (a) D. Bandyopadhyay, S. Mukherjee, L. C. Turrubiartes and B. K. Banik, Ultrason. Sonochem., 2012, 19, 969 CrossRef CAS PubMed; (b) B. Das and N. Chowdhury, J. Mol. Catal. A: Chem., 2007, 263, 212 CrossRef CAS; (c) N. Azizi, R. Baghi, H. Ghafuri, M. Bolourtchian and M. Hashemi, Synlett, 2010, 3, 379 CrossRef; (d) J. Nath and M. K. Chaudhuri, Catal. Lett., 2009, 133, 388 CrossRef CAS.
- T. C. Wabnitz and J. B. Spencer, Org. Lett., 2003, 5, 2141 CrossRef CAS PubMed.
- M. L. Kantam, V. Neeraja, B. Kavita, B. Neelima, M. K. Chaudhuri and S. Hussain, Adv. Synth. Catal., 2005, 347, 763 CrossRef CAS.
- (a) N. Srivastava and B. K. Banik, J. Org. Chem., 2003, 68, 2109 CrossRef CAS PubMed; (b) M. M. Hashemi, B. Eftekhari-Sis, A. Abdollahifar and B. Khalili, Tetrahedron, 2006, 62, 672 CrossRef CAS.
- A. K. Verma, P. Attri, V. Chopra, R. K. Tiwari and R. Chandra, Monatsh. Chem., 2008, 139, 1041 CrossRef CAS.
- A. Y. Rulev, Russ. Chem. Rev., 2011, 80, 197 CrossRef CAS.
- G. Stork, A. Brizzolara, H. Landesman, J. Szmuszkovicz and R. Terrell, J. Am. Chem. Soc., 1963, 85, 207 CrossRef CAS.
- (a) L. Rulíšek, P. Šebek, Z. Havlas, R. Hrabal, P. Čapek and A. Svatoš, J. Org. Chem., 2005, 70, 6295 CrossRef PubMed; (b) J. T. Manka, A. G. Douglass, P. Kaszynski and A. C. Friedli, J. Org. Chem., 2000, 65, 5202 CrossRef CAS PubMed.
- N. E. Leadbeater, S. J. Pillsbury, E. Shanahan and V. A. Williams, Tetrahedron, 2005, 61, 3565 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra46789a |
| This journal is © The Royal Society of Chemistry 2014 |