Synthesis of meso/macroporous aluminum nitrides via aluminum alloy ceramization
Abstract
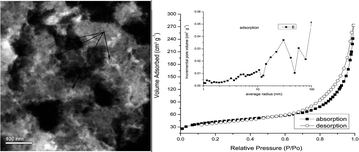
A novel and simple route, nitridization/ceramization of aluminum alloys, was introduced to produce meso/macroporous AlN. The meso/macroporous AlN were characterized by X-ray powder diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and scanning transmission electron microscopy (STEM) and N2 adsorption/desorption isotherm at −195.7 °C. Results show that pores with diameters of 2 nm to hundreds of nanometers are distributed inside the materials. The surface areas of the materials can be as large as 142.9 m2 g−1 and the cumulative volume of pores with average radii between 1 nm and 100 nm is 0.40 cm3 g−1. The formation mechanism of pores was suggested to be that the pores would be left when the Mg3N2 was removed in nitridization products of Al and Mg alloy particles.

 Please wait while we load your content...
Please wait while we load your content...