Amorphous RuO2 coated on carbon spheres as excellent electrode materials for supercapacitors
Zhou Zhou,
Yirong Zhu,
Zhibin Wu,
Fang Lu,
Mingjun Jing and
Xiaobo Ji*
Key Laboratory of Resources Chemistry of Nonferrous Metals, Ministry of Education, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China. E-mail: xji@csu.edu.cn; Fax: +86 731-88879616; Tel: +86 731-88879616
First published on 6th January 2014
Abstract
Significant enhancement in supercapacitor performances was achieved by the first fabrication of amorphous RuO2 hybrid by utilising carbon spheres as templates. The as-prepared carbon spheres (Cs)/RuO2 and reduced carbon spheres (rCs)/RuO2 composites exhibit high specific capacitances of 387 F g−1 (44.2 wt% RuO2 loading) and 614 F g−1 (72.6 wt% RuO2 loading) at a current density of 1 A g−1 at 2 mg cm−2 loading mass, showing remarkable rate capability with capacitances of 336 F g−1 and 491 F g−1 at a current density of 20 A g−1 and excellent cycling stability with capacity retention of 97.7% and 90.8% for 5000 cycles. The enhanced electrochemical performances could be attributed to the unique structure of the resulted composites as well as the high utilization of well-dispersed RuO2 nanoparticles on the carbon surface. These results demonstrate the great potential of carbon sphere-based composites in the development of high-performance electrode materials for supercapacitors.
Introduction
The diminishing availability of fossil fuels and worsening global warming issues have become major concerns nowadays, and thus the search for renewable and sustainable resources would be considered indispensable to the current world. To resolve the shortage of resources and relieve the burden on the environment, extensive efforts were made by researchers to develop various energy conversion and storage devices, especially batteries and electrochemical capacitors (ECs).1–5 As the ECs possess high power capability, excellent reversibility and long cycle life when compared with batteries, they are considered promising for various applications especially in transportation such as electric vehicles and hybrid electric vehicles.6–9 Generally, according to the charge storage mechanisms, ECs can be classified into two types. One is the electrochemical double-layer capacitor (EDLC), mainly focusing on carbon-based active materials with high surface areas, which stores energy through electrostatic forces existed in the formed double layers. While the other is pseudocapacitor, often utilizing transition metal oxides or electrically conducting polymers as the electrode materials, which works by the principle of fast and reversible faradic redox reactions.10–13Transition metal oxides have been widely used in ECs as active electrode materials due to their good conductivity and excellent power density. Among them, ruthenium oxide (RuO2), especially amorphous RuO2 has been regarded as the most potential electrode material for use in ECs owing to its good conductivity, large specific capacitance and highly reversible redox reactions. However, the observed electrochemical performance is far from its theoretical property.14–18 The most important reason is that the high surface energy of RuO2 nanoparticles could lead to severe aggregation, bringing some difficulties in the ion and electron transportation which would increase the electrical resistance. In addition, RuO2 as a noble transition metal oxide would largely raise the cost of the whole capacitor. Thereby, the research for RuO2-based composite has focused on reducing the used amount of RuO2 and achieving their outstanding pseudo-capacitive behaviour due to the compositing effects at the same time.19–22 Herein, RuO2/carbon composites have been employed widely because carbon and carbon-based materials could significantly improve the utilization efficiency of RuO2.19,23–26
To enhance the specific capacitance, various carbon and carbon-based materials have been widely applied to fabricate this composite electrode.12,27–30 Carbon spheres (Cs) are considered as ideal ones because of their superior physical and chemical properties31–34. Firstly, the Cs are obviously advantageous as components in the composites due to the ease of processability and relatively inert electrochemistry. Secondly, the surface of Cs has a large number of oxygen functional groups which could supply many electrocatalytic active sites, making it easy for a variety of redox reactions. Thirdly, the cost of Cs is relatively low and Cs are environment-friendly as a special kind of carbon-based materials. To the best of our knowledge, there have been no reports about the application of carbon sphere/RuO2 composites in supercapacitor.
In this work, a simple sol–gel method is used to synthesize Cs/RuO2 and reduced Cs/RuO2 (rCs/RuO2) hybrid as electrode materials and they are tested in a three-electrode system. The results show that the Cs/RuO2 and rCs/RuO2 have excellent electrochemical performances with remarkable specific capacitance, ultrahigh rate property and rather high cycling stability, which demonstrate that a suitable carbon-compositing strategy is an effective way to improve the electrochemical performances of ruthenium oxide as electrode used in supercapacitors.
Experiment section
Materials and chemicals
Pt foil (0.1 mm thick, 99.99%, 25 × 25 mm) was obtained from Alfa-Aesar and RuCl3·xH2O was purchased from Sinopharm Chemical Reagent Beijing Co., Ltd. Glucose and sodium hydroxide were supplied by Sinopharm Chemical Reagent Beijing Co., Ltd. All reagents were of analytical grade and subjected without any further treatment.Preparation of carbon spheres
In a typical experiment,31 8 g glucose was dissolved in 40 mL distilled water to form a clear solution. Then the solution was sealed in a 60 mL Teflon-lined autoclave and maintained at 180 °C for 4 h. The dark products were centrifuged, washed with ethanol and distilled water for several times, then dried at 80 °C for 4 h under vacuum.Preparation of Cs/RuO2 and rCs/RuO2 hybrid
The Cs/RuO2 hybrid was prepared by a sol–gel method. Newly prepared carbon spheres were dispersed in distilled water and ethanol with the help of ultrasonication for 2 hours to form a dark suspension. Then a required amount of RuCl3·xH2O was added to the reaction media to obtain a desired concentration (0.1 M), and the mixed solvent was stirred vigorously for 30 min. Then 0.3 M NaOH was slowly added to the mixture to adjust the pH to 7.0. Then the solution was kept stirring overnight. Dark products could be obtained after the filteration and wash with distilled water for several times. The resulted particles were dried at 60 °C for 10 h and calcined in air at 150 °C for 2 h. To get rCs/RuO2, the precursor of carbon spheres calcined in argon atmosphere at 400 °C for 2 h, and the subsequent procedures were the same as described above35. For comparison, RuO2 powders were prepared without Cs or rCs by the same method.Characterization of the samples
X-ray diffraction (XRD) patterns of the samples were carried out on a Rigaku D/max 2550 VB+ 18 kW X-ray diffractometer with alpha radiation at a scanning rate of 0.1°2θ s−1. Transmission electron microscopy (TEM, JEM-2100F) was applied to characterize the sample morphologies. Thermogravimetric analysis (TGA) data were collected on a thermal analysis instrument (NETZSCH STA449F3) at a heating rate of 10 °C min−1 from room temperature to 800 °C in air.Electrochemical testing
The electrochemical measurements including cyclic voltammetry (CV) and galvanostatic current charge–discharge (CD) were carried out by a Modulab (Solartron Analytical) electrochemical workstation in a three-electrode cell. The obtained samples, Cs/RuO2 (or rCs/RuO2) were mixed with acetylene black, and polyvinylidene difluoride (PVDF) with a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Then the mixture was ground uniformly to form the coating slurry by dropping into N-methyl-2-pyrrolidone (NMP). This slurry was extracted by using a micropipette and dropped onto the platinum sheet to serve as working electrode after being dried in a vacuum oven at 50 °C overnight. The loading mass of active materials was around 2.0 mg cm−2. In addition, platinum foil was used as counter electrode, saturated calomel electrode as reference electrode, and an aqueous solution of H2SO4 (1 M) as the electrolyte, respectively.
10. Then the mixture was ground uniformly to form the coating slurry by dropping into N-methyl-2-pyrrolidone (NMP). This slurry was extracted by using a micropipette and dropped onto the platinum sheet to serve as working electrode after being dried in a vacuum oven at 50 °C overnight. The loading mass of active materials was around 2.0 mg cm−2. In addition, platinum foil was used as counter electrode, saturated calomel electrode as reference electrode, and an aqueous solution of H2SO4 (1 M) as the electrolyte, respectively.
Results and discussion
Structure and morphological analysis
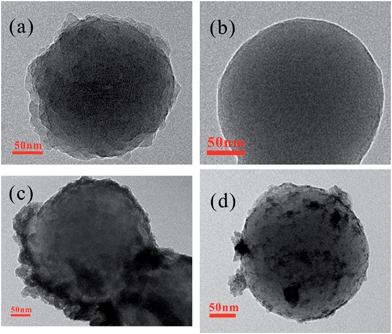
Fig. 1 (a–d) displays the morphological structure of Cs, rCs, Cs/RuO2 and rCs/RuO2, respectively. The TEM image of Cs (Fig. 1a) clearly suggests that the carbon spheres obtained by hydrothermal method from glucose at 180 °C for 4 h is uniform with a diameter about 100–200 nm, while the surface of rCs observed from TEM image (Fig. 1b) seems more smooth indicating that rCs are fitter as templates. Fig. 1c shows the TEM micrograph of the obtained Cs/RuO2 hybrid for a nominal film deposition with a diameter of 30–40 nm, from which the nanoparticle clusters on Cs could be clearly observed. It is clearly suggested that RuO2 has formed a uniform shell coated on the surface of the carbon sphere template. For comparison, the TEM image of rCs/RuO2 (Fig. 1d) manifests that the layer of small particles attached on the surface are more uniform and dispersible with a shell thickness about 20 nm.X-ray diffraction and thermogravimetric analysis
Fig. 2a and b displays the powder XRD patterns of the as-prepared Cs, rCs, Cs/RuO2 and rCs/RuO2, respectively. The low and broad diffraction peaks observed from the as-prepared Cs and rCs illustrate the amorphous structure. For the Cs/RuO2 and rCs/RuO2, the diffraction peak corresponding to the as-prepared Cs, rCs almost disappears and no crystalline RuO2 diffraction peaks are observed, revealing that the RuO2 is well coated on the surface of carbon and the RuO2 is typically amorphous due to the low annealing temperature of 150 °C. In order to determine the content of RuO2 in the as-prepared samples, a thermogravimetric analysis (TGA) was carried out in a flow of air gas with a heating rate of 10 °C min−1 to 800 °C for RuO2, Cs/RuO2 and rCs/RuO2 (Fig. 2c). The results show that the weight loss of as-prepared RuO2 commences to happen all the time while the loss before 450 °C could be mainly due to the removal of crystalline water and some residues absorbed on the surface of the products. Furthermore, the subsequent slower weight loss should be attributed to an amorphous phase rather than crystalline of the as-prepared RuO2.19,36 The total weight loss of RuO2 is about 8% in the range of 30–800 °C. For rCs/RuO2 and Cs/RuO2, the first weight loss step in the range of 30–300 °C is ascribed to the loss of absorbed water, crystalline water, and oxygen-containing groups, and the second weight loss step in the range of 300–450 °C is mostly attributed to the burning of carbon sketch of rCs or Cs. Under the tested conditions, the total weight loss of rCs/RuO2 is about 35.4%, and thus the content of hydrous RuO2 in rCs/RuO2 hybrid is about 72.6%. Moreover, the total weight loss of Cs/RuO2 is about 63.8%, presenting 44.2% content of hydrous RuO2 in Cs/RuO2 hybrid.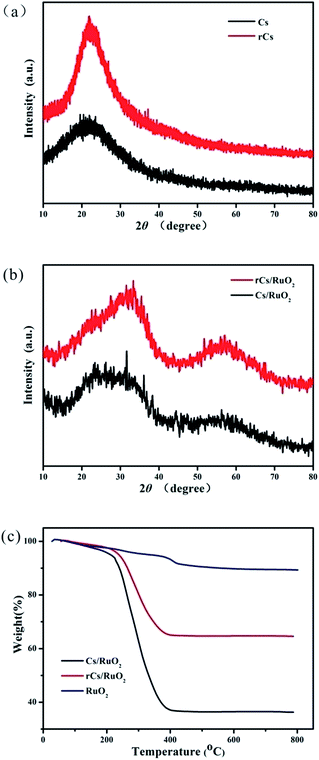 | ||
| Fig. 2 Powder XRD patterns of Cs and rCs (a), Cs/RuO2 and rCs/RuO2 (b), TGA curves of RuO2, Cs/RuO2 and rCs/RuO2 hybrid (c). | ||
Electrochemical properties analysis
| Cm = (I × △t)/(△V × m) | (1) |
| RuOx(OH)y + δH+ + δe− → RuOx−δ(OH)y+δ | (2) |
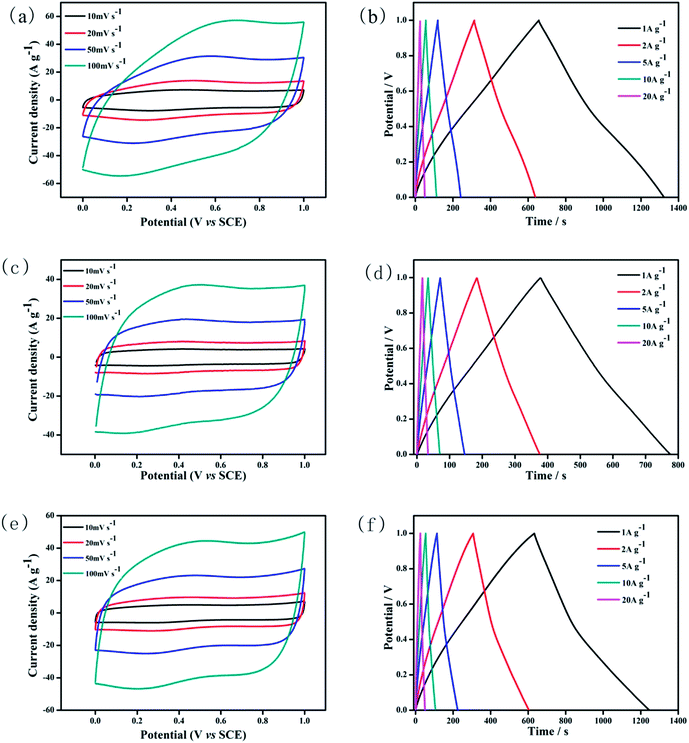
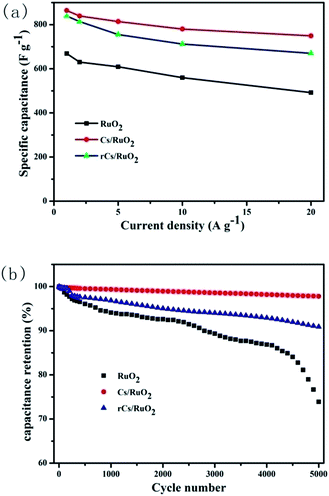
Fig. 4b displays the galvanostatic charge–discharge curves of pure RuO2. The almost triangular shape indicates its ideal capacitive behaviour because of the high degree of symmetry in charge and discharge. The specific capacitance of pure RuO2 electrode is 669, 630, 609, 560 and 492 F g−1 at current densities of 1, 2, 5, 10 and 20 A g−1, respectively. Fig. 4d exhibits the galvanostatic charge–discharge curves of Cs/RuO2 hybrid. The specific capacitances of the Cs/RuO2 electrode at current densities of 1, 2, 5, 10 and 20 A g−1 are 387, 376, 365, 350 and 336 F g−1, respectively. For rCs/RuO2 hybrid, the galvanostatic charge–discharge curves have been shown in Fig. 4f. The specific capacitances of the rCs/RuO2 electrode at current densities of 1, 2, 3, 5, 10 and 20 A g−1 are 614, 596, 553, 522 and 491 F g−1, respectively. The comparison of the specific capacitances of Cs/RuO2, rCs/RuO2 and pure RuO2 are demonstrated in Fig. 5a. It can be clearly noted that the specific capacitance of RuO2 in rCs/RuO2 hybrid is also improved as high as 839 F g−1 at 1 A g−1 when the weight of only RuO2 in this hybrid is used to calculate.
It can be clearly know that the specific capacitance of RuO2 in Cs/RuO2 hybrid is greatly improved up to 864 F g−1 at 1 A g−1 when the percentage of RuO2 in hybrid is considered. Meanwhile, the results have shown that the capacity retention rate of the Cs/RuO2 and rCs/RuO2 hybrid is 86.8% and 80.0%, which is higher than pure RuO2 (73.5%). This improvement of electrochemical properties is ascribed to the significantly enhanced utilization of RuO2 achieved by the well-dispersed RuO2 nanoparticles on Cs or rCs surface. It is known to all that an excellent cyclability of the utilized electrode material is a very important quality required for its application in supercapacitors. Thus, the Cs/RuO2 and rCs/RuO2 hybrids were tested to evaluate the cycle stability by galvanostatic charge–discharge measurements for 5000 cycles at a high current density of 5 A g−1 within a voltage range between 0 and 1 V in 1 M H2SO4 solution. The cycling performances of Cs/RuO2 and rCs/RuO2 hybrid are presented in Fig. 5b, while the cycling property of pure RuO2 is also displayed for comparison. The exhibited results could indicate the rather excellent cycling performances of Cs/RuO2 and rCs/RuO2 hybrid, from which the capacitances of Cs/RuO2 and rCs/RuO2 hybrid have retained 97.7% and 90.8% after 5000 cycles, respectively. Note that, the coulombic efficiency of Cs/RuO2 and rCs/RuO2 hybrid remains at almost 100% during the cycling process. However, the capacitance retention of pure RuO2 after 5000 cycles is just 73.9%, as shown in Fig. 5b. Thereby, it could be concluded that the presence of Cs and rCs in the RuO2-based hybrid would be able to greatly improve the cycling stability.
Conclusions
In summary, we have developed a simple sol–gel method to firstly synthesize Cs/RuO2 and rCs/RuO2 hybrid with uniform size distribution. The utilization of RuO2 is found to be significantly enhanced by coating its nanoparticles onto the surface of Cs or rCs. The investigation for the electrochemical properties has clearly shown that the unique structure of Cs/RuO2 and rCs/RuO2 composites could achieve high specific capacitance, ultrahigh rate capability and excellent cycling stability. The specific capacitances of Cs/RuO2 and rCs/RuO2 hybrid could reach 336 F g−1 and 491 F g−1 at an ultrahigh current density of 20 A g−1 in H2SO4 solution, respectively. They also have exhibit excellent capacity retention of 97.7% and 90.8% after 5000 charge–discharge cycles. These results demonstrate the potential use of Cs/RuO2 and rCs/RuO2 hybrid electrode materials for high-performance energy storage systems.The work is financially supported by the National Natural Science Foundation of China (51134007, 21003161 and 21350110326), the Distinguished Young Scientists of Hunan Province (13JJ1004), the Program for the New Century Excellent Talents in University (NCET-11-0513), the Hunan Provincial Innovation Foundation for Postgraduate (CX2013B048), and the Open-End Fund for the Valuable and Precision Instruments of Central South University (CSUZC2013004).
Notes and references
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- L. L. Zhang and X. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- J. R. Miller and P. Simon, Science, 2008, 321, 651–652 CrossRef CAS PubMed.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245–4270 CrossRef CAS.
- T. Kim, G. Jung, S. Yoo, K. S. Suh and R. S. Ruoff, ACS Nano, 2013, 7, 6899–6905 CrossRef CAS PubMed.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- Y. Wang, Z. Shi, Y. Huang, Y. Ma, C. Wang, M. Chen and Y. Chen, J. Phys. Chem. C, 2009, 113, 13103–13107 CAS.
- S. Biswas and L. T. Drzal, Chem. Mater., 2010, 22, 5667–5671 CrossRef CAS.
- B. Kang and G. Ceder, Nature, 2009, 458, 190–193 CrossRef CAS PubMed.
- W. Deng, X. Ji, Q. Chen and C. E. Banks, RSC Adv., 2011, 1, 1171–1178 RSC.
- Y. Zhang, H. Feng, X. Wu, L. Wang, A. Zhang, T. Xia, H. Dong, X. Li and L. Zhang, Int. J. Hydrogen Energy, 2009, 34, 4889–4899 CrossRef CAS PubMed.
- M. Zhi, C. Xiang, J. Li, M. Li and N. Wu, Nanoscale, 2013, 5, 72–88 RSC.
- P.-C. Chen, G. Shen, Y. Shi, H. Chen and C. Zhou, ACS Nano, 2010, 4, 4403–4411 CrossRef CAS PubMed.
- M. T. Brumbach, T. M. Alam, P. G. Kotula, B. B. McKenzie and B. C. Bunker, ACS Appl. Mater. Interfaces, 2010, 2, 778–787 CAS.
- R. Vellacheri, V. K. Pillai and S. Kurungot, Nanoscale, 2012, 4, 890–896 RSC.
- R. Borgohain, J. Li, J. P. Selegue and Y.-T. Cheng, J. Phys. Chem. C, 2012, 116, 15068–15075 CAS.
- H. Xia, Y. S. Meng, G. Yuan, C. Cui and L. Lu, Electrochem. Solid-State Lett., 2012, 15, A60–A63 CrossRef CAS PubMed.
- C.-M. Chuang, C.-W. Huang, H. Teng and J.-M. Ting, Compos. Sci. Technol., 2012, 72, 1524–1529 CrossRef CAS PubMed.
- R.-R. Bi, X.-L. Wu, F.-F. Cao, L.-Y. Jiang, Y.-G. Guo and L.-J. Wan, J. Phys. Chem. C, 2010, 114, 2448–2451 CAS.
- Z. S. Wu, D. W. Wang, W. Ren, J. Zhao, G. Zhou, F. Li and H. M. Cheng, Adv. Funct. Mater., 2010, 20, 3595–3602 CrossRef CAS.
- J. B. Goodenough and K.-S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- M. V. Reddy, G. V. Subba Rao and B. V. R. Chowdari, Chem. Rev., 2013, 113, 5364–5457 CrossRef CAS PubMed.
- M. G. Hahm, A. Leela Mohana Reddy, D. P. Cole, M. Rivera, J. A. Vento, J. Nam, H. Y. Jung, Y. L. Kim, N. T. Narayanan and D. P. Hashim, Nano Lett., 2012, 12, 5616–5621 CrossRef CAS PubMed.
- C. Liu, Z. Yu, D. Neff, A. Zhamu and B. Z. Jang, Nano Lett., 2010, 10, 4863–4868 CrossRef CAS PubMed.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- N. Soin, S. S. Roy, S. K. Mitra, T. Thundat and J. A. McLaughlin, J. Mater. Chem., 2012, 22, 14944–14950 RSC.
- Y. Zhu, X. Ji, C. Pan, Q. Sun, W. Song, L. Fang, Q. Chen and C. E. Banks, Energy Environ. Sci., 2013, 6, 3665–3675 CAS.
- Y. F. Liu, Z. H. Jiang and G. H. Yuan, Adv. Mater. Res., 2013, 608, 1074–1077 Search PubMed.
- M. V. Kiamahalleh, S. H. S. Zein, G. Najafpour, S. A. SATA and S. Buniran, Nano, 2012, 7, 1230002–1230028 CrossRef.
- H. An, Y. Wang, X. Wang, N. Li and L. Zheng, J. Solid State Electrochem., 2010, 14, 651–657 CrossRef CAS.
- X. Sun and Y. Li, Angew. Chem., Int. Ed., 2004, 43, 597–601 CrossRef PubMed.
- T.-T. Fang, H.-B. Lin and J.-B. Hwang, J. Am. Ceram. Soc., 1990, 73, 3363–3367 CrossRef CAS.
- X. Sun, J. Liu and Y. Li, Chem. – Eur. J., 2006, 12, 2039–2047 CrossRef CAS PubMed.
- C. S. Park, J. H. Kim and Y. J. Park, J. Electroceram., 2013, 31, 224–230 CrossRef CAS.
- X. W. Lou, Y. Wang, C. Yuan, J. Y. Lee and L. A. Archer, Adv. Mater., 2006, 18, 2325–2329 CrossRef CAS.
- C.-C. Hu, K.-H. Chang, M.-C. Lin and Y.-T. Wu, Nano Lett., 2006, 6, 2690–2695 CrossRef CAS PubMed.
- W. Song, X. Ji, W. Deng, Q. Chen, C. Shen and C. E. Banks, Phys. Chem. Chem. Phys., 2013, 15, 4799–4803 RSC.
- W. Deng, X. Ji, M. Gómez-Mingot, F. Lu, Q. Chen and C. E. Banks, Chem. Commun., 2012, 48, 2770–2772 RSC.
| This journal is © The Royal Society of Chemistry 2014 |