A ternary Er3+-BiVO4/TiO2 complex heterostructure with excellent photocatalytic performance
Abstract
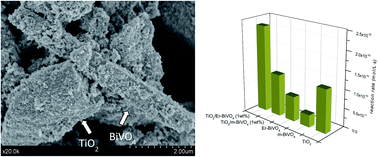
Ternary erbium doped BiVO4/TiO2 complexes are synthesized by means of a simple impregnation method with good photoactivities under sun-like excitation for the degradation of phenol. From the structural and morphological characterization it has been stated that the presence of Er3+ induces a slight stabilization of the tetragonal phase probably due to its incorporation in the BiVO4 lattice. Therefore a ternary heterostructured material has been obtained. The best photocatalytic performance was attained for the samples with 1 wt% of Er3+-doped BiVO4 content with respect to TiO2. The occurrence of a complex structural mixture with the adequate band position leads to effective charge pair separation which induces higher photocatalytic activities.

 Please wait while we load your content...
Please wait while we load your content...