Zinc iodide: a mild and efficient catalyst for one-pot synthesis of aminoindolizines via sequential A3 coupling/cycloisomerization†
Subhajit Mishraa,
Avik Kumar Bagdia,
Monoranjan Ghosha,
Subrata Sinhab and
Alakananda Hajra*a
aDepartment of Chemistry, Visva-Bharati (A Central University), Santiniketan 731235, India. E-mail: alakananda.hajra@visva-bharati.ac.in; Fax: +91 3463 261526; Tel: +91 3463 261526
bIntegrated Science Education and Research Centre, Visva-Bharati, Santiniketan 731 235, India
First published on 24th December 2013
Abstract
ZnI2 was found to be an efficient catalyst for the synthesis of indolizine derivatives by a three-component coupling of pyridine-2-carboxaldehyde/quinoline-2-carboxaldehyde, secondary amines, and terminal alkynes in high yields. This protocol is compatible with a wide range of substrates and is expected to find broad applications due to its operational simplicity, shorter reaction time and low cost. A preliminary photophysical study showed that synthesized morpholinopyrrolo[1,2-a]quinolines represent a new class of fluorophore with high quantum yield.
Introduction
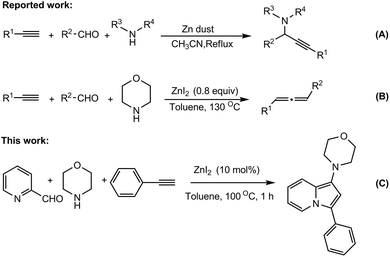
One-pot catalytic conversion of organic reactions with readily available, non-toxic, and inexpensive reagents has attracted significant research attention in recent years. Multicomponent reactions (MCRs) involve simple and flexible combination of three or more building units in a accessible one-pot reaction to form a product containing substantial elements of all the reactants.1 The multicomponent synthesis of heterocycles allows structural diversity and complexity in a single operation and atom-efficient manner. So the MCRs become an important tool for both combinatorial chemistry and diversity-oriented synthesis (DOS), and play a significant role in the development of methodology for drug discovery.2,3Indolizine is an important heterocycle, composed of an electron-rich pyrrole and an electron-poor pyridine, which has drawn considerable attention in past few years.4 The indolizine nucleus is an important skeleton for many bioactive compounds, some of them are naturally occurring,5 while others are only synthetically accessible and also important in pharmaceuticals. Indolizines and their derivatives have been allied with a broad range of biological activities such as antibacterial, antiviral, anti-inflammatory6 and central nervous system (CNS) depressant activity.7 They also serve as phosphatase inhibitors,8 aromatase inhibitors,9 antioxidant reagents,10 and calcium entry blockers11 (Fig. 1).
As a consequence, great attention has been drawn to the synthesis of indolizine derivatives and a number of elegant methods have been developed in recent years.12 In most of the cases, transition metals are used to synthesize these moieties via C–C and C–N bond forming reactions. Three-component coupling reaction of 2-pyridine carboxaldehyde, secondary amine, and terminal alkyne (A3) is one of the efficient strategies for the synthesis of these scaffolds.13 However only few methodologies have been reported based on A3 coupling reactions. But coinage metal based catalysts are used in almost all cases.13a,b,d,e Moreover, gold and silver catalysts are very expensive and additionally, sometimes inert atmosphere is required to carry out these transformations. Therefore, finding a new methodology for the synthesis of indolizines in terms of efficiency, operational simplicity and economic practicability is highly desirable.
Zinc salts are abundant, cheap, non-toxic, and exhibit environmentally benign properties. Therefore, organic chemists have been interested in using zinc salts as catalysts during the last three decades.14 Various multicomponent reactions involving alkynes have been carried out employing zinc salts in recent times.15 Kantam et al.15a reported a one-pot synthesis of propargylamines catalyzed by Zn-dust (Scheme 1, eq. A). Ma et al.15b reported ZnI2-mediated synthesis of disubstituted allenes by a three-component coupling reaction involving alkynes, amines and aldehydes (Scheme 1, eq. B). Few cycloisomerization reactions catalyzed by Zn have also been reported for the synthesis of varieties of heterocycles.16 Moreover, our own research found that Zn-salts are to be very efficient catalysts in promoting various chemical transformations.17 Based on our own research experiences in Zn catalysis and C–H bond functionalization,18 we thought that Zn could catalyze the three-component coupling and subsequent cycloisomerization to provide indolizine moiety. Herein we are pleased to report a simple and convenient approach to synthesize 1-aminoindolizines by a three-component coupling (A3) of 2-pyridine carboxaldehyde, secondary amines, and terminal alkynes using ZnI2 as a catalyst in toluene at 100 °C (Scheme 1, eq. C).
Results and discussion
We started our study by choosing pyridine-2-carboxaldehyde (1a), morpholine (2a), and phenylacetylene (3a) as the model substrates for this cascade reaction using 10 mol% ZnI2 as the catalyst in toluene for 1 hour at 60 °C. To our delight, the desired product was obtained in 62% yield. Inspired by this result, we carried out the reaction employing various zinc salts in different solvents as well as varying temperature and the results are summarized in Table 1. When the temperature was increased to 80 °C and 100 °C, the yields of the reaction increased to 75% and 92% respectively (Table 1, entries 2, and 3). However, further increasing the temperature (under reflux) the yield of the reaction decreased to 82% (Table 1, entry 4). This may be due to the decomposition of the product at higher temperature. Other Zn salts like ZnCl2, ZnBr2 and Zn(OTf)2 were not so effective like ZnI2 (Table 1, entries 5–7). Toluene appeared to be the best choice among the common solvents such as EtOH, CH3CN, 1,4-dioxane, THF, DMF, DMSO (Table 1, entries 8–13). 10 mol% ZnI2 was necessary as the optimum amount of catalyst and increasing the amount of catalyst (15 mol%) did not improve the yield, (Table 1, entry 14) whereas decreasing the amount of catalyst (5 mol%) decreased the yield (Table 1, entry 15). Use of inert atmosphere was not beneficial for this transformation. However other Lewis acids such as anhydrous AlCl3 (10 mol%) and FeCl3 (10 mol%) (Table 1, entries 16, and 17) did not afford the corresponding products. Finally optimized reaction conditions was achieved using pyridine-2-carboxaldehyde (1 mmol), morpholine (1.1 mmol) and phenylacetylene (1.2 mmol) in presence of 10 mol% of zinc iodide in toluene at 100 °C (Table 1, entry 3) under ambient air.| Entry | Catalyst | Mol% | Solvent | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1 mmol of 1a, 1.1 mmol of 2a and 1.2 mmol of 3a in the presence of catalyst in solvent (3 mL) for 1 h.b Isolated yields. | |||||
| 1 | ZnI2 | 10 | Toluene | 60 | 62 |
| 2 | ZnI2 | 10 | Toluene | 80 | 75 |
| 3 | ZnI2 | 10 | Toluene | 100 | 92 |
| 4 | ZnI2 | 10 | Toluene | Reflux | 82 |
| 5 | ZnCl2 | 10 | Toluene | 100 | 72 |
| 6 | ZnBr2 | 10 | Toluene | 100 | 75 |
| 7 | Zn(OTf)2 | 10 | Toluene | 100 | 73 |
| 8 | ZnI2 | 10 | Ethanol | Reflux | — |
| 9 | ZnI2 | 10 | Acetonitrile | Reflux | — |
| 10 | ZnI2 | 10 | 1,4-Dioxane | 100 | Trace |
| 11 | ZnI2 | 10 | THF | Reflux | Trace |
| 12 | ZnI2 | 10 | DMF | 100 | <5 |
| 13 | ZnI2 | 10 | DMSO | 100 | <5 |
| 14 | ZnI2 | 15 | Toluene | 100 | 90 |
| 15 | ZnI2 | 5 | Toluene | 100 | 78 |
| 16 | FeCl3 | 10 | Toluene | 100 | — |
| 17 | AlCl3 | 10 | Toluene | 100 | — |
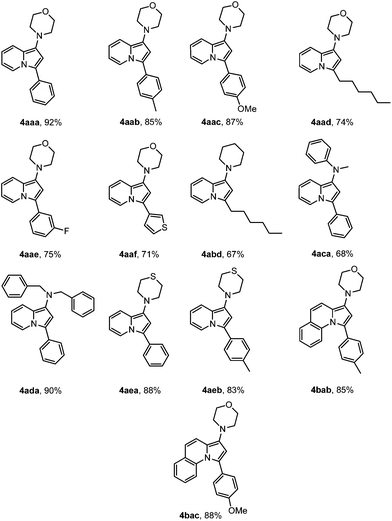
Keeping the optimized reaction conditions in hand a series of experiments were performed to verify the general applicability of the present methodology, which are summarized in Table 2. Various phenylacetylene having different substituents such as –Me (4aab, 4aeb, 4bab), –OMe (4aac, 4bac), –F (4aae) led to the corresponding products in good to excellent yields. Aliphatic alkyne like 1-octyne gave the corresponding indolizines with moderate yields (4aad, 4abd). It might be due to the instability of the compounds which decomposed quickly during column chromatography. Heteroaryl alkyne, 3-ethynyl thiophene (4aaf) also reacted under the present reaction conditions without any difficulties. Various cyclic and acyclic secondary amines, such as piperidine (4abd), dibenzylamine (4ada), and N-methylaniline (4aca) successfully gave the desired product with moderate to excellent yields. Interestingly, thiomorpholine afforded the indolizine derivatives (4aea, 4aeb) in high yields without deactivation of catalyst. Moreover, quinoline-2-carboxaldehyde was also reacted successfully under the optimized reaction conditions to give the corresponding pyrrolo[1,2-a]quinoline derivatives (4bab, 4bac).
| a Reactions conditions: 1 mmol of 1, 1.1 mmol of 2 and 1.2 mmol of 3 in the presence of ZnI2 (10 mol%) in 3 mL of toluene at 100 °C for 1 h. Isolated yields. |
|---|
 |
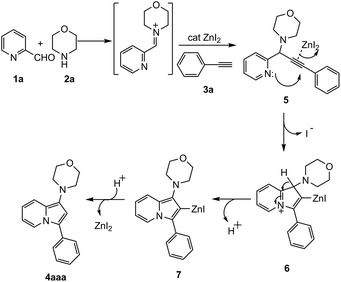
A proposed mechanism for this cyclization reaction is represented in Scheme 2. The first step is the A3 coupling of pyridine-2-carboxaldehyde, morpholine and phenylacetylene in presence of catalytic amount of zinc iodide to form the corresponding propargylic pyridine 5 through the iminium intermediate.15b Zn co-ordinates with the triple bond of alkyne to enhance the electrophilicity. As a result nucleophilic attack by lone pair on nitrogen atom takes place resulting the formation of 6 which on subsequent deprotonation and demetallation giving rise to the corresponding 1-amino indolizine.
Organic compounds exhibiting intense fluorescence are always in demand as these are used as biological markers,19 functional organic devices and sensors,20 dyes21 as well as in organic light emitting devices (OLEDs). On seeing the long conjugation of pyrrolo[1,2-a]quinoline moiety, we took interest in doing photophysical study. Therefore, a preliminary survey of the photophysical activity of few compounds (4aaa, 4bab, 4bac) was carried out (see, ESI†). The absorption spectra and emission spectra of 4bac were recorded in various solvents (Fig. 2). In absorption spectra, there is no significant shifting. The characteristic emission band was observed with the maximum varying between 514 and 541 nm. This morpholinopyrrolo[1,2-a]quinoline fluorophore exhibited high quantum yield (0.47) in chlorobenzene and also showed large Stokes shift which is an attractive property for biological probes. Moreover a distinct bathochromic (red) shift of the emission maxima was observed with increasing the polarity of the solvents. This solvatochromic behaviour which results from the stabilization of polar emitting state by polar solvents is characteristic for molecules showing internal charge transfer on excitation.22
 | ||
| Fig. 2 Absorbance and fluorescence emission spectra of 4bac in various solvents (concentration ∼ 10−4 mol L−1). Hex = hexane, Ben = benzene, CB = chlorobenzene, ACN = acetonitrile. | ||
Conclusions
In summary, we have developed a facile and economic protocol for the one-pot synthesis of the biologically potent indolizine derivatives by a three-component coupling of pyridine-2-carboxaldehyde/quinoline-2-carboxaldehyde, secondary amines, and terminal alkynes. The key features of the present protocol are wide substrate scope, operational simplicity, shorter reaction times, low cost of the catalyst and good to excellent isolated yields. We believe that our present protocol is the simplest and a convenient way to synthesize indolizine scaffolds. Furthermore, morpholinopyrrolo[1,2-a]quinolines represent a new class of fluorophore with high quantum yields.Acknowledgements
A. H. acknowledges the financial support from the Department of Science and Technology, Govt. of India (Grant no. SR/S5/GC-05/2010). We are thankful to DST-FIST and UGC-SAP. S. M. and M. G. thank UGC, New Delhi, India and A. K. B. thanks CSIR, New Delhi, India for their fellowship.Notes and references
- (a) Multicomponent Reactions, ed. J. Zhu and H. Bienayme, Wiley-VCH, Weinheim, 2005 Search PubMed; (b) A. Domling, Chem. Rev., 2006, 106, 17 CrossRef PubMed; (c) V. Nair, C. Rajesh, A. U. Vinod, S. Bindu, A. R. Sreekanth, J. S. Mathen and L. Balagopal, Acc. Chem. Res., 2003, 36, 899 CrossRef CAS PubMed; (d) D. Tejedor and F. Garcia-Tellado, Chem. Soc. Rev., 2007, 36, 484 RSC; (e) C. Simon, T. Constantieux and J. Rodriguez, Eur. J. Org. Chem., 2004, 4957 CrossRef CAS; (f) D. J. Ramon and M. Yus, Angew. Chem., Int. Ed., 2005, 44, 1602 CrossRef CAS PubMed; (g) N. Mizuno and M. Misono, Chem. Rev., 1998, 98, 199 CrossRef CAS PubMed; (h) S. Maiti, S. Biswas and U. Jana, J. Org. Chem., 2010, 75, 1674 CrossRef CAS PubMed; (i) S. Santra, A. K. Bagdi, A. Majee and A. Hajra, RSC Adv., 2013, 3, 24931 RSC.
- (a) J. D. Sunderhaus, C. Dockendorff and S. F. Martin, Org. Lett., 2007, 9, 4223 CrossRef CAS PubMed; (b) C. Hulme and V. Gore, Curr. Med. Chem., 2003, 10, 51 CrossRef CAS; (c) F. Lieby-Muller, T. Constantieux and J. Rodriguez, J. Am. Chem. Soc., 2005, 127, 17176 CrossRef CAS PubMed; (d) R. W. Armstrong, A. P. Combs, P. A. Tempest, S. D. Brown and T. A. Keating, Chem. Res., 1996, 29, 123 CrossRef CAS; (e) V. A. Chebanov, V. E. Saraev, S. M. Desenko, V. N. Chernenko, I. V. Knyazeva, U. Groth, T. N. Glasnov and C. O. Kappe, J. Org. Chem., 2008, 73, 5110 CrossRef CAS PubMed.
- (a) B. Willy and T. J. Muller, Eur. J. Org. Chem., 2008, 4157 CrossRef CAS; (b) S. L. Dax, J. J. McNally and M. A. Youngman, Curr. Med. Chem., 1999, 6, 255 CAS; (c) M. M. Heravi, B. Baghernejad, H. A. Oskooie and R. Hekmatshoar, Tetrahedron Lett., 2008, 49, 6101 CrossRef CAS PubMed; (d) N. M. Evdokimov, A. S. Kireev, A. A. Yakovenko, M. Y. Antipin, I. V. Magedov and A. Kornienko, J. Org. Chem., 2007, 72, 3443 CrossRef CAS PubMed; (e) A. Dömling, Comb. Chem. High Throughput Screening, 1998, 1, 1 Search PubMed.
- (a) The Structure, Reactions, Synthesis, and Uses of Heterocyclic Compounds, in Comprehensive Heterocyclic Chemistry, ed. A. R. Katritzky and C. W. Rees, Pergamon Press: Oxford, 1984, vol. 1–8 Search PubMed; (b) W. Flitsch, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Rees and E. F. V. Scriven, Pergamon; Oxford, U.K., 1996, vol. 8, p. 237 Search PubMed.
- (a) J. P. Micheal, Nat. Prod. Rep., 2002, 19, 742 RSC; (b) J. P. Micheal, Alkaloids, 2001, 55, 91 Search PubMed.
- (a) V. R. Vemula, S. Vurukonda and C. K. Bairi, Int. J. Pharm. Sci. Rev. Res., 2011, 11, 159 CAS; (b) G. S. Singh and E. E. Mmatli, Eur. J. Med. Chem., 2011, 46, 5237 CrossRef CAS PubMed.
- W. B. Harrell, J. Pharm. Sci., 1970, 59, 275 CrossRef CAS.
- C. Narajji, M. D. Karvekar and A. K. Das, Asian J. Chem., 2008, 20, 6183 CAS.
- T. Weide, L. Arve, H. Prinz, H. Waldmann and H. Kessler, Bioorg. Med. Chem. Lett., 2006, 16, 59 CrossRef CAS PubMed.
- O. B. Østby, B. Dalhus, L.-L. Gundersen, F. Rise, A. Bast and G. R. M. Haenen, Eur. J. Org. Chem., 2000, 3763 CrossRef.
- C. Poty, V. Gibon, G. Evrard, B. Norberg, D. P. Vercauteren, J. Gubin, P. Chatelain and F. Durant, Eur. J. Org. Chem., 1994, 29, 911 CAS.
- (a) M. T. Gandaségui and J. Alvarez-Builla, J. Chem. Res., 1986, 74 Search PubMed; (b) I. V. Seregin, A. W. Schammel and V. Gevorgyan, Org. Lett., 2007, 9, 3433 CrossRef CAS PubMed; (c) Y. Liu, Z. Song and B. Yan, Org. Lett., 2007, 9, 409 CrossRef CAS PubMed; (d) I. V. Seregin and V. Gevorgyan, J. Am. Chem. Soc., 2006, 128, 12050 CrossRef CAS PubMed; (e) D. Chernyak, S. B. Gadamsetty and V. Gevorgyan, Org. Lett., 2008, 10, 2307 CrossRef CAS PubMed; (f) J. Barluenga, G. Lonzi, L. Riesgo, L. A. López and M. Tomás, J. Am. Chem. Soc., 2010, 132, 13200 CrossRef CAS PubMed; (g) D. Chernyak, C. Skontos and V. Gevorgyan, Org. Lett., 2010, 12, 3242 CrossRef CAS PubMed; (h) U. Bora, A. Saikia and R. C. Boruah, Org. Lett., 2003, 5, 435 CrossRef CAS PubMed.
- (a) B. Yan and Y. Liu, Org. Lett., 2007, 9, 4323 CrossRef CAS PubMed; (b) B. K. Bai, J. Zeng, J. Ma, B. K. Gorityala and X.-W. Liu, J. Comb. Chem., 2010, 12, 696 CrossRef PubMed; (c) S. S. Patil, S. V. Patil and V. D. Bobade, Synlett, 2011, 2379 CAS; (d) S. Mishra, B. Naskar and R. Ghosh, Tetrahedron Lett., 2012, 53, 5483 CrossRef CAS PubMed; (e) M. Albaladejo, F. Alonso and M. Yus, Chem.–Eur. J., 2013, 19, 5242 CrossRef CAS PubMed.
- (a) X.-F. Wu and H. Neumann, Adv. Synth. Catal., 2012, 354, 3141 CrossRef CAS; (b) D. Kundu, S. Ahammed and B. C. Ranu, Green Chem., 2012, 14, 2024 RSC; (c) R. Kumar, R. Thilagavathi, R. Gulhane and A. K. Chakraborti, J. Mol. Catal. A: Chem., 2006, 250, 226 CrossRef CAS PubMed.
- (a) M. L. Kantam, V. Balasubrahmanyam, K. B. S. Kumar and G. T. Venkanna, Tetrahedron Lett., 2007, 48, 7332 CrossRef CAS PubMed; (b) J. Kuang and S. Ma, J. Am. Chem. Soc., 2010, 132, 1786 CrossRef CAS PubMed.
- (a) A. Sniady, A. Durham, M. S. Morreale, K. A. Wheeler and R. Dembinski, Org. Lett., 2007, 9, 1175 CrossRef CAS PubMed; (b) P. Aschwanden, D. E. Frantz and E. M. Carreira, Org. Lett., 2000, 2, 2331 CrossRef CAS PubMed; (c) M. Nakamura, C. Liang and E. Nakamura, Org. Lett., 2004, 6, 2015 CrossRef CAS PubMed; (d) K. Alex, A. Tillack, N. Schwarz and M. Beller, Org. Lett., 2008, 10, 2377 CrossRef CAS PubMed.
- (a) A. Roy, M. Rahman, S. Das, D. Kundu, S. K. Kundu, A. Majee and A. Hajra, Synth. Commun., 2009, 39, 590 CrossRef CAS; (b) D. Kundu, A. Majee and A. Hajra, J. Heterocyclic Chem., 2009, 46, 1019 CrossRef; (c) D. Kundu, A. Majee and A. Hajra, J. Chin. Chem. Soc., 2008, 55, 1186 CAS; (d) S. K. Kundu, A. Majee and A. Hajra, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2009, 48, 408 Search PubMed.
- (a) M. Rahman, A. K. Bagdi, A. Majee and A. Hajra, Tetrahedron Lett., 2011, 52, 4437 CrossRef CAS PubMed; (b) A. K. Bagdi, M. Rahman, S. Santra, A. Majee and A. Hajra, Adv. Synth. Catal., 2013, 355, 1741 CrossRef CAS.
- (a) V. S. Padalkar and N. Sekar, Curr. Chem. Lett., 2012, 1, 1 CAS; (b) M. L. Capobianco, G. Barbarella and A. Manetto, Molecules, 2012, 17, 910 CrossRef CAS PubMed; (c) H. Kobayashi, M. Ogawa, R. Alford, P. L. Choyke and Y. Urano, Chem. Rev., 2010, 110, 2620 CrossRef CAS PubMed.
- (a) Y. Sun, L. Duan, D. Zhang, J. Qiao, G. Dong, L. Wang and Y. Qiu, Adv. Funct. Mater., 2011, 21, 1881 CrossRef CAS; (b) M. T. Sharbati and F. Emami, Opt. Express, 2011, 19, 3619 CrossRef CAS PubMed; (c) S. W. Chiu, L.-Y. Lin, H.-W. Lin, Y.-H. Chen, Z.-Y. Huang, Y.-T. Lin, F. Lin, Y.-H. Liu and K.-T. Wong, Chem. Commun., 2012, 48, 1857 RSC.
- (a) M. D. Bowman, M. M. Jacobson and H. E. Blackwell, Org. Lett., 2006, 8, 1645 CrossRef CAS PubMed; (b) Y. Araki, A. Andoh, Y. Fujiyama, K. Hata, J. Makino, T. Okuno, F. Nakanura and T. Bamba, J. Chromatogr., Biomed. Appl., 2001, 753, 209 CrossRef CAS.
- (a) R. Lartia, C. Allain, G. Bordeau, F. Schmidt, C. Fiorini-Debuisschert, F. Charra and M.-P. Teulade-Fichou, J. Org. Chem., 2008, 73, 1732 CrossRef CAS PubMed; (b) S. Achelle, A. Barsella, C. Baudequin, B. Caro and F. R.-l. Guen, J. Org. Chem., 2012, 77, 4087 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, characterization data, and NMR spectra for all compounds. See DOI: 10.1039/c3ra46513f |
| This journal is © The Royal Society of Chemistry 2014 |