Surface synergism of an Ag–Ni/ZrO2 nanocomposite for the catalytic transfer hydrogenation of bio-derived platform molecules†
Abstract
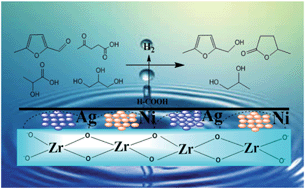
Levulinic acid was completely and selectively converted to GVL, in the presence of formic acid over an Ag–Ni/ZrO2 catalyst. The synergism between Ag and Ni in transfer hydrogenation eliminates the need for external hydrogen, making the process safer. The magnetic nature of the catalyst offers easy recovery for efficient recycling. This approach is standardized for the hydrogenation of several C3–C6 platform molecules in an aqueous medium.

 Please wait while we load your content...
Please wait while we load your content...