Simultaneous removal of NOX and SO2 with H2O2 over Fe based catalysts at low temperature†
Abstract
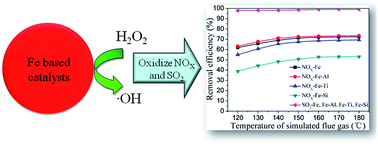
Simultaneous flue gas desulfurization and denitrification are achieved with ˙OH radicals generated from H2O2 catalyzed by Fe based transition metal (Fe, Fe–Al, Fe–Ti, Fe–Si) oxides in the duct, followed by purification with ammonia-based glass made washing tower. The main recovered products are the ammonia nitrate and sulfate, which are important fertilizers. In this oxidation-removal process, 100% SO2 and 74% NOX removal efficiencies are achieved at the simulated flue gas temperature of 120–180 °C under the applied conditions with a space velocity of 36 000 h−1. The experimental results show that the enhancement of Lewis acid benefits the generation of O2 without ˙OH radical production. The increase of Fe3+ bonded with hydroxyl group and points of zero charge value are beneficial to produce ˙OH radicals, thus improving the NOX removal efficiency.

 Please wait while we load your content...
Please wait while we load your content...