Systematic stability investigation of perfluorosulfonic acid membranes with varying ion exchange capacities for fuel cell applications†
Abstract
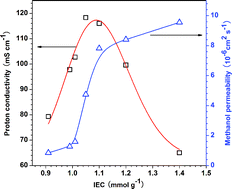
The stability of perfluorosulfonic acid (PFSA) membranes is one of the most essential issues affecting the performance of fuel cells. The investigation of membrane stability is therefore of significant importance for fuel cell applications. So far, however, the most elaborate research on membrane stability has focused on the commercial Nafion® series, which has a fixed ion exchange capacity (IEC) of 0.91 mmol g−1; there is therefore a lack of systematic studies for membranes with varying IEC values. Recently, our group successfully synthesized a variety of PFSA resins with IEC values ranging from 0.91 to 1.4 mmol g−1, providing an opportunity for us to investigate the effect of factors such as the IEC value, crystallinity and ion-cluster channels, on membrane stability. The proton conductivity and methanol permeability of the membranes are also considered to be crucial parameters which affect fuel cell performance. With these in mind, optimized membranes suitable for fuel cell applications were finally identified.

 Please wait while we load your content...
Please wait while we load your content...