A simple one-step solution deposition process for constructing high-performance amorphous zirconium oxide thin film†
Abstract
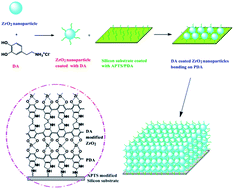
Based on the magic substance dopamine (DA), a simple and effective one-step solution deposition strategy has been developed for constructing high-performance amorphous zirconium oxide (ZrO2) nanocomposite thin film. DA can be auto-polymerized to form a polydopamine (PDA) coating, which adheres strongly to the silicon substrate and serves as an active platform to induce the subsequent deposition of ZrO2. The obtained ZrO2 nanocomposite thin films with the thickness of about one hundred nanometers presents outstanding mechanical and tribological performances even without a subsequent high-temperature annealing process. More importantly, the preparation process is definitely low-consumption and environmentally friendly. This work overcomes the claim that ZrO2 films obtained by aqueous solution deposition process generally possess low mechanical properties and thus avoid the defects of focusing mainly on nature and performances of heat-treated and crystalline ZrO2. Based on this work, it is believed that the as-deposited ZrO2 nanocomposite film can be widely used as a protecting coating and lubricating material for micro- and nano-electromechanical systems (MEMS/NEMS) and many other systems working in similar conditions.

 Please wait while we load your content...
Please wait while we load your content...