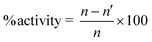DOI:
10.1039/C3RA43201G
(Paper)
RSC Adv., 2014,
4, 6068-6073
The synthesis of amino acid derived glycoconjugates and the investigation of their anti-inflammatory and analgesic properties†
Received
25th June 2013
, Accepted 20th November 2013
First published on 21st November 2013
Abstract
A series of 4,6-O-ethylidene-β-D-glucopyranosylamine derived glycoconjugates containing amino- and aromatic acids have been synthesized. All these molecules have been tested for their anti-inflammatory and analgesic activity on Wistar rat and Swiss Albino mice respectively. The anti-inflammatory studies were explored using a carrageenan induced paw oedema model while an acetic acid induced writhing model was adapted for the analgesic studies. All of the compounds exhibited anti-inflammatory and analgesic activity in the range of 63–84% and 86–94% respectively.
Introduction
Carbohydrates, naturally occurring chiral compounds, are one of the major sources of energy for living organisms and are required in the formation of polysaccharides, nucleic acids and antibiotics. Their association with proteins lead to glycoprotein chemistry, which controls various life processes in the form of collagens, mucins, transferrin, immunoglobins, various hormones, enzymes, antifreeze proteins etc.1–6 In most glycoproteins, glucose is covalently linked with a polypeptide unit via either O- or N-glycosylation.2,3 Inspired from biological importance, we have developed a new receptor molecule, N-(2-hydroxybenzoyl)-L-alanyl-4,6-O-ethylidene-β-D-glucopyranosylamine (C1; Fig. 1) containing a sugar and an amino acid linked together by an amide bond.4,5 C1 exhibits the selective sensing ability of cupric acetate among similar salts of other transition metal ions as well as different salts (NO3−, Cl−, SO42−, OTf− and ClO4−) of Cu(II) itself.7 We also succeed in establishing its crystal structure and interaction with the free and protein-bound tryptophan residue using UV-visible, fluorescence and mass spectroscopy.8 The versatile nature of C1 prompted us to find its application in biological system as it interacts with free and protein bound selective amino acid residues and Cu(II) ions, which is the third most abundant transition metal ion in biological systems. Several glycoconjugates including peptide derivatives have been used as anti-inflammatory agents9–19 whereas salicylic acid derivatives have been used as an analgesic.20–24
 |
| | Fig. 1 The structure of N-(2-hydroxybenzoyl)-L-alanyl-4,6-O-ethylidene-β-D-glucopyranosylamine (C1). | |
Since, our molecule possesses all these basic fragments like sugar, amino acid and salicylic acid; it is expected that it should be a good candidate to exhibit anti-inflammatory and analgesic properties in an in vivo system. In order to explore these activities, we synthesized a series of five new similar molecules as shown in Scheme 1.
 |
| | Scheme 1 The synthetic route of compounds C1–C6; ECF, ethylchloroformate; TEA, triethylamine; EDCI, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide; HOBT, hydroxybenzotriazole. | |
We tested our compounds on male Wister rats for anti-inflammatory activity following a carrageenan induced paw oedema model13,15,25–29 and the results were compared with a standard drug, Indomethacin. The analgesic studies were performed on Swish Albino mice following an acetic acid induced writhing test model26–29 and the results were compared with Aspirin. All of the compounds exhibited both an anti-inflammatory and analgesic behaviour, and the alanine containing molecules were shown to be the best for both activities. Hence, this paper deals with the synthesis of a series of new chiral glycoconjugates and their applications as anti-inflammatory and analgesic agents.
Results and discussion
Anti-inflammatory studies
Natural as well as synthetic compounds possessing glycosidic linkages have been widely used as anti-inflammatory agents.12,14,16,30–32 Salicylate containing plants have been used for the production of anti-inflammatory drug since the eighteenth century.21,22,33,34 All these literature reports prompted us to investigate the anti-inflammatory behaviour of C1 as it possess units of sugar, amino acids and salicylic acid. We screened the anti-inflammatory effect of C1 using a carrageenan induced paw oedema model as described in the Experimental section and the result was exciting. We were able to notice a reasonable amount of anti-inflammatory effect. Inspired from these preliminary results, we synthesized a series of five new compounds using 4,6-O-ethylidene-β-D-glucopyranosylamine but varying both amino- and aromatic acids. The compounds were characterized using FTIR, NMR and mass spectroscopy as mentioned in the Experimental section. After confirming the purity of compounds C2–C6 using 1H and 13C NMR, we tested all these compounds for an anti-inflammatory activity following the procedure mentioned in Experimental section and the results are summarized in Table 1. We used 100 mg of each compound per kg wt of rat and the result was compared with marketed drug Indomethacin. All the compounds exhibited a good amount of activity, and the results for C3 were comparable with those obtained from 15 mg Indomethacin dose per kg weight of Wister rat. Following the same model and dose, Kumar et al. have reported an anti-inflammatory activity in the range of 68–74% using four different amino acid derivatives of Diclofenac and a 1 h duration.29 Anikina et al. have developed three amino acid derivatives of ibuprofen and found anti-inflammatory activity in the range of 14–39% after 4 h using an 87.2 mg dose.35 Hence, our finding is better than both of these previous reports. Several more reports are available, however they have followed either a different protocol for in vivo studies or use different doses.18,36,37
Table 1 The anti-inflammatory activities of C1–C6 on male Wister rats
| Treatment group |
Dose (mg kg−1, i.p.) |
Mean decrease in paw volume (mL) ± SEMa |
% reduction in inflammatory symptoms |
| Indicates 0.01 > P relative to the control. |
| Carrageenan control |
— |
0.503 ± 0.062 |
— |
| C1 |
100 |
0.183 ± 0.054 |
63.6 |
| C2 |
100 |
0.138 ± 0.052 |
72.5 |
| C3 |
100 |
0.080 ± 0.034 |
84.1 |
| C4 |
100 |
0.135 ± 0.047 |
73.2 |
| C5 |
100 |
0.140 ± 0.045 |
72.2 |
| C6 |
100 |
0.143 ± 0.037 |
71.5 |
| Indomethacin |
15 |
0.075 ± 0.015 |
85.1 |
Analgesic studies
Natural products which exhibit anti-inflammatory activities have also been used to cure pain.21,34 Several drug molecules containing peptides20,38,39 and salicylic acid derivatives20,37 have been used as analgesic agents. Inspired from this literature background, we tested all six molecules as analgesic agents on mice following the acetic acid induced writhing test model as mentioned in the Experimental section. The dose of each compound was 100 mg kg−1 of mice weight and the activity was calculated using the equation given below:| |
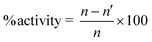 | (1) |
where n = mean number of writhes of control group (vehicle-injected mice) and n′ = mean number of writhes of test group mice. A summary of the results is presented in Fig. 2, which clearly infer the analgesic effects induced by the test compounds. All of our compounds exhibited a better activity than the marketed drug Aspirin. The data were analyzed by a one way ANOVA post hoc test Dunnet, p < 0.01, n = 6 per group and the results are expressed as the mean. Using the same model and dose Kumar et al.29 have reported the analgesic activity in the range of 28–51% while Balamani and Sekar20 found the same in the range of 66–100%. In these reports the former used four different amino acid derivatives of Diclofenac whereas the latter utilized amino acid and peptide derivatives of Aspirin. Anikina et al.35 used same model together with a 302.5 mg kg−1 dose of three amino acid derivatives of ibuprofen and obtained an activity in the range of 66–87%. Levit et al.26 studied the analgesic activity using several amino acid derivatives of Naproxen using a model similar to ours but using a 94 mg kg−1 mice dosage. They obtained an analgesic activity in the range of 3–83% and hence, our results are either better or comparable with the available literature reports.
 |
| | Fig. 2 A bar diagram revealing the analgesic results. *P < 0.01 with respect to the control. | |
Conclusions
Recently we had developed an alanyl glycoconjugate derived novel receptor C1, which selectively recognises cupric acetate among similar salts of other transition metal ions as well as different salts (NO3−, Cl−, SO42−, OTf− and ClO4−) of Cu(II) itself.7 It also selectively recognises the free and protein-bound tryptophan residue.8 Inspired from these versatile results, we have synthesized five new similar chiral compounds and explored their application as anti-inflammatory and analgesic agents in an in vivo system. Anti-inflammatory studies were performed on Wistar rats using the carrageenan induced paw oedema model, whereas the analgesic studies were executed on Swiss Albino mice by adapting an acetic acid induced writhing test model. All of our compounds exhibited both, anti-inflammatory and analgesic behaviours, and the alanine containing molecules yielded the best results in both of the activities. We found anti-inflammatory and analgesic activities in the range of 63–84% and 86–94% respectively.
Experimental
Materials and methods
All experiments were performed under normal atmospheric conditions and room temperature. Salicylic acid, methyl salicylic acid and indole acid were purchased from Aldrich and the rest of the chemicals along with the solvent was procured from local suppliers. The solvents were purified by standard methods. Compounds F1, A1 and C1 were prepared according to our earlier report.4 The IR spectra were recorded on an Shimadzu IR Prestige-21 spectrometer and the NMR spectra were recorded on a Bruker (300 MHz) spectrometer in DMSO-d6. The chemical shifts are given in δ (ppm) and the coupling constants in Hz. Mass spectra were obtained on a ‘Hewlett-Packard’ HP GS/MS 5890/5972 mass spectrometer data system using argon/xenon (6 kV, 10 mA).
Animals
Male Albino mice (25–35 g) and male Wistar rats (210–260 g) were obtained from Hissar agricultural University, Hissar, Haryana, India. Animal experimentation was conducted in adherence to the Institutional Animal Ethics Committee of Birla Institute of Technology and Science, Pilani, Rajasthan, India (Protocol no. IAEC/RES/14/01, dated 07.09.11). The animals were housed in standard polycarbonate cages and maintained under standard light (lights on from 7:00 A.M. to 7.00 P.M.), temperature (23 ± 2 °C), and room humidity (50–60%) conditions. Each treatment group consisted of 6–8 randomly chosen animals. The animals were given free access to food (standard pellet feed) and filtered water. In order to prevent habituation effects the animals were used only once for each experiment.
N-Fmoc-L-phenylalanyl-4,6-O-ethylidene-β-D-glucopyranosylamine (F2)
This compound was prepared following the procedure adopted for F1, but using 4,6-O-ethylidene-β-D-glucopyranosylamine (3.18 g, 15.51 mmol), Fmoc phenylalanine acid (6.00 g, 15.50 mmol), ethylchloroformate (1.48 mL, 15.55 mmol) and triethylamine (2.16 mL, 15.52 mmol). Yield: 5.00 g (56%); white solid; mp 193–197 °C; IR (KBr): 3340, 1674, 1531, 1089, 1012, 889, 752 cm−1; 1H NMR (DMSO-d6, 300 MHz, ppm): δ 8.81 (1H, br, amide NH), 7.86 (2H, d, J = 7.6 Hz, Fmoc Ar-H), 7.63–7.62 (2H, m, Fmoc Ar-H), 7.41–7.13 (10H, m, amide NH, Fmoc and Phe Ar-H), 5.38 (2H, br, glucose OH's), 4.85 (1H, t, J = 8.9 Hz, glucose H-1), 4.69 (1H, q, J = 5.1 Hz, ethylidene CH), 4.36 (1H, br, Fmoc CH), 4.27 (1H, m, Phe chiral CH), 4.09 (2H, m, Fmoc CH2), 3.99 (1H, m, glucose H-5), 3.39 (2H, m, glucose H-3, H-6a), 3.22 (2H, m, glucose H-4, H-6b), 3.11 (1H, t, J = 9.2 Hz, glucose H-2), 2.92 (1H, m, Phe CH2a), 2.72 (1H, m, Phe-CH2b), 1.21 (3H, d, J = 4.8 Hz, ethylidene CH3). 13C NMR (DMSO-d6, 75 MHz, ppm): >CONH2: 175.88, 143.23; Ar (Phe and Fmoc): 140.08, 139.51, 138.09, 130.05, 129.60, 128.72, 127.97, 126.70, 122.07, 120.72, 110.48; ethylidene CH: 99.23; glucose: 81.09, 80.92, 74.03, 73.98, 68.24, 68.05; chiral CH: 57.10; Ph-CH2: 41.08; Fmoc-CH2: 38.49; ethylidene CH3: 20.99. MS (ESI) m/z calcd for C32H34N2O8 (M + H)+ 574.6; found 575.0.
L-Phenylalanyl-4,6-O-ethylidene-β-D-glucopyranosylamine (A2)
This compound was prepared following the procedure adopted for A14 but using F2 (2.65 g, 4.62 mmol) and a 25% piperidine/DMF solution (1.5 mL). Yield: 1.05 g (65%); white solid; mp 188–190 °C; IR (KBr): 3358, 1681, 1537, 1091, 1012, 889, 702 cm−1; 1H NMR (DMSO-d6, 300 MHz, ppm): δ 8.52 (1H, d, J = 8.9 Hz, amide NH), 7.26–7.15 (5H, m, Phe Ar-H), 5.37 (2H, br, glucose OH's), 4.82 (1H, t, J = 8.2 Hz, glucose H-1), 4.68 (1H, q, J = 5.0 Hz, ethylidene CH), 3.98 (1H, m, glucose H-5), 3.36 (3H, m, glucose H-3, H-6a, Phe chiral CH), 3.20 (2H, m, glucose H-4, H-6b), 3.10 (1H, t, J = 9.2 Hz, glucose H-2), 2.90 (1H, m, Phe CH2a), 2.52 (1H, m, Phe-CH2b), 1.64 (2H, br, NH2), 1.22 (3H, d, J = 4.9 Hz, ethylidene CH3). 13C NMR (DMSO-d6, 75 MHz, ppm): >CONH2: 175.88; Ar (Phe): 139.50, 130.05, 128.73, 126.70; ethylidene CH: 99.23; glucose: 80.93, 80.89, 74.02, 73.98, 68.24, 68.04; Phe chiral CH: 57.09; Phe CH2: 41.46; ethylidene CH3: 21.00. MS (ESI) m/z calcd for C17H24N2O6 (M + H)+ 352.4; found 353.0.
N-(2-Indoloyl)-L-alanyl-4,6-O-ethylidene-β-D-glucopyranosylamine (C2)
This compound was prepared following the procedure adopted for C1, but using A1 (0.09 g, 0.33 mmol), indole-2-carboxylic acid (0.05 g, 0.31 mmol), TEA (0.06 mL, 0.40 mmol), HOBT (0.042 g, 0.30 mmol) and EDC (0.07 g, 0.37 mmol). Yield: 0.04 g (30%); cream color solid; mp > 300 °C; IR (KBr): 3523, 3278, 3062, 1691, 1602, 1546, 1099, 748 cm−1; 1H NMR (DMSO-d6, 300 MHz, ppm): δ 11.62 (1H, s, indole NH), 8.60 (1H, d, J = 8.4 Hz, amide NH), 8.53 (1H, d, J = 8.0 Hz, amide NH), 7.60 (1H, d, J = 8.4 Hz, indole H), 7.40 (1H, d, J = 7.8 Hz, indole H), 7.24 (1H, s, indole H), 7.16 (1H, t, J = 7.6 Hz, indole H), 7.01 (1H, t, J = 7.5 Hz, indole H), 5.32 (1H, d, J = 5.6 Hz, glucose OH), 5.14 (1H, d, J = 5.2 Hz, glucose OH), 4.82 (1H, t, J = 8.8 Hz, glucose H-1), 4.67 (1H, q, J = 5.1 Hz ethylidene CH), 4.57 (1H, m, Ala chiral CH), 3.98 (1H, m, glucose H-5) 3.34 (2H, m, glucose H-3, H-6a), 3.21 (2H, m, glucose H-4, H-6b), 3.08 (1H, t, J = 8.8 Hz, glucose H-2), 1.32 (3H, d, J = 6.8 Hz, Ala CH3), 1.20 (3H, d, J = 4.8 Hz, ethylidene CH3). 13C NMR (DMSO-d6, 75 MHz, ppm): >CONH2: 173.72, 161.15; indole: 137.09, 132.02, 127.66, 124.06, 122.23, 120.37, 112.93, 104.24; ethylidene CH: 99.21; glucose: 81.05, 80.89, 74.09, 73.75, 68.27, 68.01; Ala chiral CH: 49.16; ethylidene CH3: 20.98; Ala CH3: 19.24. HRMS m/z calcd for C20H24N3O7 (M)− 418.1614; found 418.1616.
N-(2-Hydroxy-6-methylbenzoyl)-L-alanyl-4,6-O-ethylidene-β-D-glucopyranosylamine (C3)
This compound was prepared following the procedure adopted for C1, but using A1, (0.95 g, 3.44 mmol), 3-methyl salicylic acid (0.50 g, 3.29 mmol), TEA (0.60 mL, 4.25 mmol), HOBT (0.44 g, 3.29 mmol) and EDC (0.76 g, 3.96 mmol). Yield 0.55 g (41%); white solid; mp 220–222 °C (dec); IR (KBr): 3500, 3312, 3014, 1690, 1566, 1093, 746 cm−1; 1H NMR (DMSO-d6, 300 MHz, ppm): 8.80 (1H, d, J = 7.5 Hz, amide NH), 8.61 (1H, d, J = 8.8 Hz, amide NH), 7.80 (1H, d, J = 8.8 Hz, ArH), 7.29 (1H, d, J = 7.3 Hz, ArH), 6.78 (1H, t, J = 7.7 Hz, ArH), 5.32 (1H, d, J = 5.1 Hz, glucose OH), 5.11 (1H, d, J = 5.3 Hz, glucose OH), 4.83 (1H, t, J = 8.9 Hz, glucose H-1), 4.67 (1H, q, J = 4.9 Hz, ethylidene CH), 4.53 (1H, m, Ala chiral CH), 3.98 (1H, m, glucose H-5), 3.35 (2H, m, glucose H-3, H-6a), 3.20 (2H, m, glucose H-4, H-6b), 3.08 (1H, t, J = 9.0 Hz, glucose H-2), 2.13 (3H, s, Ar-CH3), 1.33 (3H, d, J = 7.2 Hz, Ala CH3), 1.21 (3H, d, J = 5.0 Hz, ethylidene CH3). 13C NMR (DMSO-d6, 75 MHz, ppm): >CONH2: 173.06, 170.35; Ar: 159.80, 135.34, 126.51, 126.04, 118.38, 114.19; ethylidene CH: 99.22; glucose: 81.01, 80.85, 74.22, 73.73, 68.28, 68.01; Ala chiral CH: 49.23; ethylidene CH3: 20.98; Ala CH3: 18.49; Ar-CH3: 16.20. HRMS m/z calcd for C19H27N2O8 (M + H)+ 411.1767; found 411.1766 and C19H26N2NaO8 (M + Na)+ 433.1587; found 433.1453.
N-(2-Hydroxybenzoyl)-L-phenylalanyl-4,6-O-ethylidene-β-D-glucopyranosylamine (C4)
This compound was prepared following the procedure adopted for C1, but using A2 (0.27 g, 0.77 mmol), salicylic acid (0.10 g, 0.72 mmol), EDC (0.17 g, 0.89 mmol), TEA (0.12 mL, 0.89 mmol) and HOBT (0.09 g, 0.67 mmol) in DMF (0.2 mL). Yield: 0.11 g (33%); white solid; mp 213–215 °C (dec); IR (KBr): 3282, 1660, 1535, 1091, 1010, 889, 754 cm−1; 1H NMR (DMSO-d6, 300 MHz, ppm): δ 11.90 (1H, br, ArOH), 8.88 (2H, m, amide NH's), 7.88 (1H, d, J = 7.8 Hz, ArH), 7.38–7.11 (6H, m, ArH), 6.86 (2H, t, J = 8.0 Hz, ArH), 5.35 (1H, br, glucose OH), 5.20 (1H, d, J = 5.0 Hz, glucose OH), 4.87 (1H, t, J = 8.9 Hz, glucose H-1), 4.81 (1H, m, Phe chiral CH), 4.70 (1H, q, J = 5.0 Hz, ethylidene CH), 4.00 (1H, m, glucose H-5), 3.4–3.05 (7H, m, Phe-CH2 + glucose H-2, H-3, H-4, H-6a,b), 1.22 (3H, d, J = 5.0 Hz, ethylidene CH3). 13C NMR (DMSO-d6, 75 MHz, ppm): >CONH2: 172.06, 167.97; Ar: 159.45, 138.26, 134.18, 129.95, 129.62, 128.70, 127.01, 119.42, 117.76, 116.73; ethylidene CH: 99.24; glucose: 81.00, 80.85, 74.26, 73.82, 68.28, 68.02; chiral CH: 54.86; Ph-CH2: 38.12; ethylidene CH3: 20.99. HRMS m/z calcd for C24H29N2O8 (M + H)+ 473.1924; found 473.1953 and C24H28N2NaO8 (M + Na)+ 495.1743; found 495.1722.
N-(2-Indoloyl)-L-phenylalanyl-4,6-O-ethylidene-β-D-glucopyranosylamine (C5)
This compound was prepared following the procedure adopted for C1, but using A2 (0.22 g, 0.63 mmol), indole-2-carboxylic acid (0.10 g, 0.62 mmol), TEA (0.11 mL, 0.79 mmol), HOBT (0.09 g, 0.59 mmol) and EDC (0.14 g, 0.73 mmol) in DMF (0.3 mL). Yield: 0.1 g (32%); white solid; IR (KBr): 3533, 3284, 1685, 1640, 1560, 1091, 1012, 887, 748 cm−1; 1H NMR (DMSO-d6, 300 MHz, ppm): 11.47 (1H, s, Indole NH), 8.84 (1H, d, J = 8.8 Hz, amide NH), 8.55 (1H, d, J = 8.8 Hz, amide NH), 7.60 (1H, d, J = 8.0 Hz, ArH), 7.37–7.33 (3H, m, ArH), 7.22–7.09 (5H, m, ArH), 7.00 (1H, m, ArH), 5.35 (1H, d, J = 5.0 Hz, glucose OH), 5.18 (1H, d, J = 6.0 Hz, glucose OH), 4.89 (1H, t, J = 9.0 Hz, glucose H-1), 4.78 (1H, m, Phe chiral CH), 4.69 (1H, q, J = 5.0 Hz, ethylidene CH), 4.00 (1H, m, glucose H-5), 3.39 (2H, m, glucose H-3, H-6a), 3.23 (2H, m, glucose H-4, H-6b), 3.12 (1H, t, J = 9.2 Hz, glucose H-2), 2.97 (2H, m, Phe CH2a,b), 1.22 (3H, d, J = 5.0 Hz, ethylidene CH3). 13C NMR (DMSO-d6, 75 MHz, ppm): >CONH2: 172.73, 161.44; Ar: 138.84, 137.06, 131.87, 129.94, 128.67, 127.62, 126.91, 124.02, 122.23, 120.36, 112.91, 103.93; ethylidene CH: 99.24; glucose: 80.97, 80.87, 74.32, 73.84, 68.28, 68.03; Phe chiral CH: 55.04; Phe CH2: 38.19; ethylidene CH3: 20.99. HRMS m/z calcd for C26H30N3O7 (M + H)+ 496.2084; found 496.2196 and C26H29N3NaO7 (M + Na)+ 518.1903; found 518.1989.
N-(2-Hydroxy-3-methyl-benzoyl)-L-phenylalanyl-4,6-O-ethylidene-β-D-glucopyranosylamine (C6)
This compound was prepared following the procedure adopted for C1, but using A2 (0.23 g, 0.65 mmol), 3-methyl salicylic acid (0.10 g, 0.65 mmol), TEA (0.12 mL, 0.86 mmol), HOBT (0.09 g, 0.67 mmol) and EDC (0.17 g, 0.89 mmol) in DMF (0.3 mL). Yield: 0.12 g (36%); white solid; mp 122–125 °C; IR (KBr): 3300, 1685, 1627, 1548, 1091, 1008, 889, 748 cm−1; 1H NMR (DMSO-d6, 300 MHz, ppm): δ 8.89 (2H, br, amide NH), 7.76 (1H, d, J = 7.2 Hz, ArH), 7.34–7.12 (6H, m, ArH), 6.77 (1H, t, J = 7.0 Hz, ArH), 5.23 (2H, br, glucose OH's), 4.88–4.70 (3H, m, ethylidene CH, glucose H-1, Phe chiral CH), 4.00 (1H, br, glucose H-5), 3.50–2.93 (7H, m, glucose H-2, H-3, H-4, H-6a,b, Phe CH2a,b), 2.08 (3H, br, Ar-CH3), 1.22 (3H, br, ethylidene CH3). 13C NMR (DMSO-d6, 75 MHz, ppm): >CONH2: 172.15, 170.60; Ar: 159.73, 138.72, 135.34, 129.83, 128.74, 126.99, 126.51, 125.77, 118.40, 113.99; ethylidene CH: 99.24; glucose: 81.03, 80.86, 74.31, 73.79, 68.29, 68.03; Phe chiral CH: 55.15; Phe CH2: 37.59; ethylidene CH3: 20.99; Ar-CH3: 16.12. HRMS m/z calcd for C25H31N2O8 (M + H)+ 487.2080; found 487.2084 and C26H30N2NaO8 (M + Na)+ 509.1900; found 509.1864.
The carrageenan-induced rat paw oedema model for anti-inflammatory activity
One day before the experiment, Wistar rats (210–260 g) were housed in a group of six per cage. The animals were starved overnight and to insure uniform hydration, the injection volume received by each rat was kept constant with respect to its body weight. The animals were divided into six groups with six animals in each group, their left paws were marked with ink at the level of the lateral malleolus and baseline paw volume (mL) was measured. Thirty minutes after the drug/vehicle administration, the rats were injected with 0.05 mL of a 1% solution of carrageenan into the plantar side of the left hind paw and the paw volume was measured plethysmographically (UGO Basile 7140 Plethesmometer) at 0, 30, 60, 90, 120 min. The mean decrease in the paw volume (mL) was taken into account at 120 min time intervals.
The acetic acid-induced writhing test for analgesic activity
All of the mice (with body weight, 25–35 g) under study were given 0.1 mL of acetic acid (0.01% v/v) intraperitoneally irrespective of their body weight after 30 min of vehicle/drug administration. The mice were placed individually into glass beakers immediately after the acetic acid injection and allowed to elapse for five min. The mice were then observed for a period of 30 min and the number of writhes (indicated by stretching of the abdomen with simultaneous stretching of at least one hind limb) was recorded for each mice. The percent inhibition was calculated as the average writhes in the control group minus the writhes in the drug group divided by the writhes in the control group times 100%. The time period with the greatest percent of inhibition was considered the peak time and was taken into account for statistical analysis.
Acknowledgements
A. K. Sah is grateful to the Department of Science and Technology (DST) and the University Grants Commission (UGC) for the financial support under SERC Fast Track and Special Assistance Programme respectively. We thank Mr Sushil Yadav and Dipali Gupta of Department of Pharmacy, BITS Pilani, Pilani campus for helping us with the in vivo studies.
Notes and references
- K. Botham, P. Mayes, R. Murray, D. Granner and V. Rodwell, Harper's Illustrated Biochemistry, The McGraw-Hill Companies, Inc., New York, 196Y208, 27th edn, 2006 Search PubMed.
- B. G. Davis, J. Chem. Soc., Perkin Trans. 1, 1999, 3215–3237 RSC.
- M. J. Grogan, M. R. Pratt, L. A. Marcaurelle and C. R. Bertozzi, Annu. Rev. Biochem., 2002, 71, 593–634 CrossRef CAS PubMed.
- B. G. Davis, Chem. Rev., 2002, 102, 579–602 CrossRef CAS PubMed.
- D. P. Gamblin, E. M. Scanlan and B. G. Davis, Chem. Rev., 2009, 109, 131–163 CrossRef CAS PubMed.
- P. Lafite and R. Daniellou, Nat. Prod. Rep., 2012, 29, 729–738 RSC.
- A. K. Sah and K. Soni, Catal. Commun., 2012, 28, 120–123 CrossRef CAS PubMed.
- K. Soni and A. K. Sah, RSC Adv., 2013, 3, 12096–12099 RSC.
- B. N. N. Rao, M. B. Anderson, J. H. Musser, J. H. Gilbert, M. E. Schaefer, C. Foxall and B. K. Brandley, J. Biol. Chem., 1994, 269, 19663–19666 CAS.
- C. R. Bertozzi and L. L. Kiessling, Science, 2001, 291, 2357–2364 CrossRef CAS.
- F. Wu, Y. Yi, P. Sun and D. Zhang, Bioorg. Med. Chem. Lett., 2007, 17, 6430–6433 CrossRef CAS PubMed.
- K. W. Woo, E. Moon, S. Y. Park, S. Y. Kim and K. R. Lee, Bioorg. Med. Chem. Lett., 2012, 22, 7465–7470 CrossRef CAS PubMed.
- A. Valdivia, Y. Perez, A. Dominguez, J. Caballero, L. Gomez, E. H. Schacht and R. Villalonga, Macromol. Biosci., 2005, 5, 118–123 CrossRef CAS PubMed.
- D. P. Iga, S. Iga and A. Craciun, Rom. Biotech. Lett., 2010, 15, 5493–5497 CAS.
- J. F. M. Leite, A. M. S. Assreuy, M. R. L. Mota, P. H. d. S. F. Bringel, R. R. Lacerda, V. d. M. Gomes, J. B. Cajazeiras, K. S. do Nascimento, H. d. L. F. Pessôa, C. A. d. A. Gadelha, P. Delatorre, B. S. Cavada and T. Santi-Gadelha, Molecules, 2012, 17, 3277–3290 CrossRef CAS PubMed.
- E. Haslam, J. Nat. Prod., 1996, 59, 205–215 CrossRef CAS PubMed.
- S. M. Rele, W. Cui, L. Wang, S. Hou, G. Barr-Zarse, D. Tatton, Y. Gnanou, J. D. Esko and E. L. Chaikof, J. Am. Chem. Soc., 2005, 127, 10132–10133 CrossRef CAS PubMed.
- S. K. Khalikov, M. Kodirov and S. Alieva, Chem. Nat. Compd., 2006, 42, 204–207 CrossRef CAS PubMed.
- S. Zhang and K. S. Mark, Microvasc. Res., 2012, 84, 161–168 CrossRef CAS PubMed.
- J. Balamani and M. Sekar, Hygeia J. D. Med., 2011, 3, 1–15 CAS.
- K. K. Wu, Circulation, 2000, 102, 2022–2023 CrossRef CAS.
- S. Tanasescu, H. Lévesque and C. Thuillez, Rev. Med. Interne, 2000, 21(suppl. 1), S18–26s CrossRef.
- S. P. Clissold, Drugs, 1986, 32, 8–26 CrossRef CAS PubMed.
- S. Higuchi, N. Tanaka, Y. Shioiri, S. Otomo and H. Aihara, Int. J. Tissue React., 1986, 8, 327 CAS.
- C. A. Winter, E. A. Risley and G. W. Nuss, Exp. Biol. Med., 1962, 111, 544–547 CrossRef CAS.
- G. L. Levit, L. V. Anikina, Y. B. Vikharev, A. M. Demin, V. A. Safin, T. V. Matveeva and V. P. Krasnov, Pharm. Chem. J., 2002, 36, 232–236 CrossRef CAS.
- A. Saha and M. Ahmed, Pak. J. Pharm. Sci., 2009, 22, 74–77 Search PubMed.
- M. Amir and K. Shikha, Eur. J. Med. Chem., 2004, 39, 535–545 CrossRef CAS PubMed.
- S. Kumar, D. K. Tyagi and A. Gupta, J. Pharm. Sci., 2010, 2, 369–375 CAS.
- G. Yuan, M. L. Wahlqvist, G. He, M. Yang and D. Li, Asia Pac. J. Clin. Nutr., 2006, 15, 143 CAS.
- C. Wang, T.-T. Zhang, G.-H. Du and D.-M. Zhang, J. Asian Nat. Prod. Res., 2011, 13, 817–825 CrossRef CAS PubMed.
- O. v. Ahsen, U. Voigtmann, M. Klotz, N. Nifantiev, A. Schottelius, A. Ernst, B. M. ler-Tiemann and K. Parczyk, Anal. Biochem., 2008, 372, 96–105 CrossRef PubMed.
- J. R. Vane and R. M. Botting, Inflammation Res., 1998, 47, 78–87 CrossRef.
- J. R. Vane and R. M. Botting, Am. J. Med., 1998, 104, 2S–8S CrossRef CAS.
- L. V. Anikina, G. L. Levit, A. M. Demin, Y. B. Vikharev, V. A. Safin, T. V. Matveeva and V. P. Krasnov, Pharm. Chem. J., 2002, 36, 237–239 CrossRef CAS.
- N. M. Divanyan, S. A. Kazaryan, L. K. Galstyan, O. L. Mndzhoyan, V. G. Sarkisyan, D. G. Chilingaryan and N. A. Apoyan, Pharm. Chem. J., 1978, 12, 1150–1153 CrossRef.
- A. Saqib, C. S. Karigar, M. A. Pasha and M. S. Harish, J. Pharm. Sci. Res. Opp., 2012, 2, 35–38 CAS.
- M. Li, L. Zhou, G. Ma and S. Dong, Regul. Pept., 2012, 179, 23–28 CrossRef CAS PubMed.
- A. Novoa, S. Van Dorpe, E. Wynendaele, M. Spetea, N. Bracke, S. Stalmans, C. Betti, N. N. Chung, C. Lemieux, J. Zuegg, M. A. Cooper, D. Tourwe, B. D. Spiegeleer, P. W. Schiller and S. Ballet, J. Med. Chem., 2012, 55, 9549–9561 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra43201g |
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here.