A one-step hydrothermal preparation strategy for layered BiIO4/Bi2WO6 heterojunctions with enhanced visible light photocatalytic activities†
Hongwei Huang*,
Shuobo Wang,
Na Tian and
Yihe Zhang*
National Laboratory of Mineral Materials, School of Materials Science and Technology, China University of Geosciences, Beijing, 100083, P.R. China. E-mail: hhw@cugb.edu.cn; Tel: +86-10-82332247
First published on 18th December 2013
Abstract
Through the introduction of a new Bi-based semiconductor BiIO4, the novel BiIO4/Bi2WO6 heterojunctions composed of two layered structures were successfully fabricated by a one-step hydrothermal method. The as-prepared samples were thoroughly characterized by XRD, SEM, TEM, HRTEM, XPS, ICP, DRS and PL spectra technologies. The photodegradation experiments indicated that the BiIO4/Bi2WO6 composites showed much higher visible-light-driven (VLD) photocatalytic activity than those of either individual BiIO4 and Bi2WO6 for rhodamine B (RhB) degradation, which are attributed to the high separation of photogenerated electron–hole pairs resulted by the BiIO4/Bi2WO6 heterojunctions. This is the first report of the photocatalytic activity of the new Bi-based compound BiIO4 and BiIO4/Bi2WO6 composites under visible light. Moreover, our research provided a new layered semiconductor, which can be applied in the future for heterojunction construction and energy band structure design.
Introduction
Photocatalytic technology has attracted much attention for offering great potential use in organic wastewater treatment for environmental remediation.1–3 Though TiO2 is the most widely researched photocatalyst, it suffers from many problems, including the poor catalytic stability, high recombination of photogenerated electron–hole pairs and especially the low solar energy conversion efficiency due to its large energy bandgap.4,5 In order to cope with these problems, great efforts have been made on the development of new photocatalysts with visible light response and improvement of the photocatalytic activity.6 Among which, fabrication of heterojunction photocatalysts by coupling of two semiconductors with appropriate band edges has been a significant approach as a novel strategy.7–9 In the heterojunction composites, the recombination of photogenerated electron–hole pairs can be effectively suppressed through a charge transfer, thus resulting in high photocatalytic activity. Nevertheless, the construction of heterojunction system is not easy because the energy levels of the two semiconductors must be well-matched overlapping band-structures. Therefore, seeking for coupling photocatalysts with matched conduction band (CB) and valence band (VB) has been the most crucial problem.Bismuth tungstate (Bi2WO6), with perovskite-like structure, is one of the simplest members and probably the most studied example of the Aurivillius oxide family. It possesses a layered structure composed of alternating bismuth oxide (Bi2O2)2+ layers and (WO4)2− octahedral layers,10 which is considered to promote the generation and the separation of the charge carriers, and thus, Bi2WO6 exhibits excellent photocatalytic activity under visible light irradiation.11,12 To further improve the photocatalytic activity of Bi2WO6, various strategies were adopted, including element doping (such as B, Fe, Eu, etc.),13–15 solid-solution design (such as Bi2WxMo1−xO6, BixY1−xWO6, etc.),16,17 forming p–n heterojunction,18 as well as coupling with heterogeneous semiconductors.19–23 Up to now, several kinds of Bi2WO6 based heterojunctions have been developed, for instance, TiO2/Bi2WO6,19 ZnWO4/Bi2WO6,20 WO3/Bi2WO6,21 BiOI/Bi2WO6 (ref. 22) and C3N4/Bi2WO6,23 etc.
BiIO4 is a new bismuth iodate as a nonlinear optical material synthesized by Nguyen et al. recently.24 It also exhibits a layered structural topology. Instead of a perovskite-like anion block separating the (Bi2O2)2+ layers in Aurivillius phases, the locally polar iodate (IO3)− anions are observed between (Bi2O2)2+ layers in the structure of BiIO4. This layered configuration will also be favorable for charge transfer. By investigating the energy levels of BiIO4, it is fortunate to find that the energy levels of BiIO4 and Bi2WO6 are well-matched overlapping band-structures. Thus, they may be suitable to construct a heterojunction with a high visible light catalytic activity.
Herein, we successfully fabricated the BiIO4/Bi2WO6 heterojunctions containing two layered structures via a one-step hydrothermal method. The photocatalytic experiments under visible light irradiation showed that the present BiIO4/Bi2WO6 heterojunction possesses excellent photocatalytic activity for degrading rhodamine B (RhB) under visible light irradiation, which is much higher than those of either individual BiIO4 or Bi2WO6. Accordingly, a reasonable model is proposed to illustrate the key roles of BiIO4 in the photocatalytic process. It is noteworthy that this is the first report regarding the photocatalytic decomposition for organic compounds of BiIO4 and BiIO4/Bi2WO6 composite under visible light.
Experimental section
Synthesis
All the reagents used were AR grade and without further purification. BiIO4/Bi2WO6 heterojunction was obtained by a hydrothermal method. In a typical procedure, 2.42 g Bi(NO3)3·5H2O was added to 30 ml deionized water, and the breaker was placed in an ultrasonic bath for 10 min to dissolve raw materials. Meanwhile, a certain amount of I2O5 and Na2WO4·2H2O were dissolved in 30 ml deionized water to obtain a clear solution. Then, the solution was added to the suspension and subsequently stirred for another 3 h at room temperature. The resulting white suspension was subsequently transferred into a 100 ml Teflon-lined stainless autoclave and heated at 180 °C for 24 h. After cooling, the products were collected by filtration and washed repeatedly with deionized water and ethanol and then dried at 60 °C for 12 h. According to this method, different molar ratios of BiIO4/Bi2WO6 at 2%, 5%, 10% and 15% were prepared, respectively. The pure BiIO4 and Bi2WO6 samples were also synthesized under the same conditions as references.Characterizations
The crystal structures of the obtained samples were examined by X-ray diffraction (XRD) using a D8 Advance X-ray diffractometer (Bruker AXS, Germany) with Cu Kα radiation (λ = 1.5418 Å). The scanning step width of 0.02° and the scanning rate of 0.2° s−1 were applied to record the patterns in the 2θ range of 10–70°. The morphology and microstructure were obtained by a S-4800 scanning electron microscope (SEM) and a transmission electron microscopy (TEM and HRTEM; JEM-2100F). X-ray photoelectron spectroscopy (XPS) analysis was performed on a VGMK II X-ray photoelectron spectrometer. A Varian 710-ES (Varian, Shanghai, China) inductively coupled plasma optical emission spectrometer (ICP-OES) with Sepex Certiprep standards was used to analyze the element of composition of the samples. UV-vis spectra were performed with sample powder from Perkin Elmer Lambda 35 UV-vis spectrometer. The spectra were collected at 200–1000 nm referenced to BaSO4. Room temperature excitation and emission spectra were measured on a JOBIN 10 YVON FluoroMax-3 fluorescence spectrophotometer with a photomultiplier tube 11 operating at 400 V, and a 150 W Xe lamp was used as the excitation lamp.Photocatalytic evaluation
Photocatalytic activities of BiIO4/Bi2WO6 heterojunctions were evaluated by degradation of RhB under visible light irradiation of a 1000 W Xenon lamp with the 400 nm cutoff filter. Powder photocatalyst (50 mg) was dispersed into 50 ml of dye solution (10−5 mol l−1). Before illumination, the photocatalyst powder and dye solution were vigorously stirred in dark for 1 h to achieve the adsorption–desorption equilibrium of suspensions. After that, the light was turned on, and 2 ml of the suspension was taken at certain intervals and separated through centrifugation. The UV-vis spectra of the centrifuged solution were recorded using a U-3010 spectrophotometer.Results and discussion
Characterization of BiIO4/Bi2WO6 heterojunctions
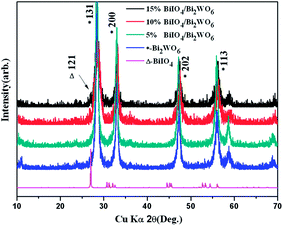
BiIO4 crystallizes in orthorhombic space group Pca2. In the asymmetric units, there are only one crystallographically independent Bi atom, one independent I atom and four independent O atoms. The Bi–O and I–O distances range between 2.246(6)–2.581(3) Å and 1.813(4)–1.844(4) Å respectively. The structure may be described as containing layers of (Bi2O2)2+ cations that are connected to (IO3)− anions. The (Bi2O2)2+ layers are structurally analogous to that observed in Bi2WO6 with Aurivillius structure. The crystal structures of Bi2WO6 and BiIO4 were shown in Fig. 1a and b, respectively. It can be seen that they possess very similar crystal structures, except the space between (Bi2O2)2+ layers were filled with WO6 octahedra and IO3 groups in Bi2WO6 and BiIO4, respectively.The XRD patterns of Bi2WO6, BiIO4 and BiIO4/Bi2WO6 heterojunctions were presented in Fig. 2. Due to the low content of BiIO4 and high intensity of Bi2WO6 diffraction peaks, only one diffraction peak of orthorhombic BiIO4 (ICSD #262019) appeared when the molar ratio of BiIO4/Bi2WO6 is from 0.1 to 0.15, and the strongest peak of BiIO4 is attributed to (121) plane, which is in good agreement with the following HRTEM analyses. No impurity peak is found in BiIO4/Bi2WO6 composites, suggesting that the heterojunction has a two-phase composition: BiIO4 and Bi2WO6.
Fig. 3 shows the SEM images of BiIO4, Bi2WO6 and BiIO4/Bi2WO6 composite photocatalysts with different molar ratios, respectively. The pure Bi2WO6 products (Fig. 3a) were composed of a number of regular nanosheets with sizes of several hundred nanometers and thicknesses below 50 nm, and the pure BiIO4 sample also displays flakelike morphologies with the size ranging from several hundred nanometers to tens of micrometers (Fig. 3f). After introducing BiIO4 onto the surface of Bi2WO6, the BiIO4/Bi2WO6 heterostructures were formed as shown in Fig. 3b–e. Though the size of Bi2WO6 in all these heterojunctions is similar to that of the pristine Bi2WO6, the morphologies of the composite became gradually irregular compared to pure Bi2WO6 nanosheets as the increase of the content of BiIO4, which indicates the formation of BiIO4/Bi2WO6 heterostructures.
 | ||
| Fig. 3 SEM images of (a) pure Bi2WO6, (b) 2% BiIO4/Bi2WO6, (c) 5% BiIO4/Bi2WO6 BiIO4, (d) 10% BiIO4/Bi2WO6, (e) 15% BiIO4/Bi2WO6 and (f) pure BiIO4. | ||
The obtained 10% BiIO4/Bi2WO6 heterojunction was further characterized by TEM and HRTEM. The low and high magnification TEM image in Fig. 4a and b confirmed that products were all composed of nanoflakes, but more irregular than pure Bi2WO6 (Fig. S1†). From Fig. 4b, it can be clearly seen that the thicknesses of these nanoflakes are estimated to be only several nanometers. The HRTEM image and fast Fourier transform (FFT) images (Fig. 4c–e) of BiIO4/Bi2WO6 heterojunction confirm the two phases BiIO4 and Bi2WO6 and their single crystal nature. The lattice resolved HRTEM image from inverse FFT indicates that the two sets of lattice fringes with spacings of 0.314 nm and 0.326 nm, which are consistent with the spacing of (131) and (121) planes of orthorhombic Bi2WO6 and BiIO4, respectively.
 | ||
| Fig. 4 (a and b) TEM, (c) HRTEM images, (d and e) FFT (fast Fourier transition) patterns and (f and g) inverse FFT (fast Fourier transition) patterns of the lattice fringe of 10% BiIO4/Bi2WO6 sample. | ||
To further confirm the BiIO4/Bi2WO6 heterojunctions, we have carried out the XPS and ICP-OES measurements on the 10% BiIO4/Bi2WO6 sample. The overall XPS spectra for the 10% BiIO4/Bi2WO6 was shown in Fig. 5a, in which the Bi, W, O and I peaks could be detected for the 10% BiIO4/Bi2WO6 composites. Fig. 5b–e are the high resolution XPS spectra of Bi 4f, W 4f, O 1s and I 3d respectively. It can be seen that, the binding energies of Bi 4f7/2, Bi 4f5/2, W 4f7/2, W 4f5/2, and O 1s were 159.4, 164.6, 35.6, 37.8, and 530.2 eV, respectively. The I 3d region exhibit the characteristic peaks at 619.5 and 631.4 eV, which were ascribed to I 3d5/2 and I 3d3/2, respectively. Moreover, the ICP results indicated the molar ratio of Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I
I![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W = 21.3
W = 21.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.8, which is in agreement with the expected value in 10% BiIO4/Bi2WO6 sample. These results all demonstrate the BiIO4/Bi2WO6 heterojunction are well formed.
9.8, which is in agreement with the expected value in 10% BiIO4/Bi2WO6 sample. These results all demonstrate the BiIO4/Bi2WO6 heterojunction are well formed.
Fig. 6 displayed the UV-vis diffuse reflectance absorption spectra (DRS) of the as-prepared BiIO4, Bi2WO6 and BiIO4/Bi2WO6 photocatalysts. Compared with pure Bi2WO6, the BiIO4/Bi2WO6 composites all exhibit blue-shifts on the absorption edges. Since the visible light absorption of Bi2WO6 was caused by band gap transition,16 the absorption vs. energy was plotted. As shown in the inset (a) of Fig. 6, the band gap of Bi2WO6 was estimated to be 2.7 eV. In semiconductors, the square of absorption coefficient is linear with energy for direct optical transitions in the absorption edge region; whereas the square root of absorption coefficient is linear with energy for indirect transitions.25 Data plots of absorption1/2 versus energy in the absorption edge region for BiIO4 shown in the inset (b) of Fig. 6 is nearly linear, which indicate the absorption edge of BiIO4 is caused by indirect transitions. Band gap of BiIO4 is determined by optical absorption near the band edge by the following equation:
| αhν = A(hν − Eg)n/2 | (1) |
 | ||
| Fig. 6 UV-vis diffuse reflectance spectra of Bi2WO6, BiIO4 and BiIO4/Bi2WO6 samples. Band gaps of Bi2WO6 (inset a) and BiIO4 (inset b). | ||
Photocatalytic study of BiIO4/Bi2WO6 heterojunctions
On the basis of the above results, the photodegradation of RhB has been investigated to evaluate the photocatalytic activity of as-synthesized BiIO4/Bi2WO6 heterojunctions under visible light irradiation. The characteristic absorption peak at 554 nm was employed to determine the degradation degree of RhB. As displayed in Fig. 7a, the blank experiment (without photocatalysts) shows that RhB molecules are very stable and the photolysis is negligible. As for the pure BiIO4 and Bi2WO6, they show relatively poor activity, on which approximately 10% and 55% of MO are decomposed after irradiation for 3 h. When BiIO4 and Bi2WO6 were combined to construct BiIO4/Bi2WO6 heterostructures, it can be found that the content of BiIO4 dramatically affects the photocatalytic activities of BiIO4/Bi2WO6 even though the content of BiIO4 was very low. The photocatalytic activities of BiIO4/Bi2WO6 heterojunctions with molar ratio 2%, 5% and 10% is significantly improved compared with pure BiIO4 and Bi2WO6, while it decreases with the further increase of BiIO4 content. When the theoretical molar ratio of BiIO4 to Bi2WO6 was 10%, the highest photocatalytic activity was obtained, resulting in the degradation efficiency of RhB 95.5% after 3 h irradiation. As shown in Fig. 7b, the main absorption peak of RhB molecules at 554 nm decreases with irradiation time, and almost disappears after about 3 h. In order to compare the degradation rate quantitatively, the first order kinetics curves (Fig. S2†) and RhB photodegradation apparent rate constants (Fig. 7c) were also plotted. The experimental data obviously showed the apparent rate constant k is 0.032 h−1, 0.38 h−1, 0.56 h−1, 1.10 h−1, 0.23 h−1 and 0.27 h−1 for pure BiIO4, 2% BiIO4/Bi2WO6, 5% BiIO4/Bi2WO6, 10% BiIO4/Bi2WO6, 15% BiIO4/Bi2WO6 and pure Bi2WO6, respectively. In other words, 10% BiIO4/Bi2WO6 exhibits the highest photocatalytic activity, which is almost 34.4 and 4.1 times higher than those of pure BiIO4 and Bi2WO6, respectively, which suggests that BiIO4/Bi2WO6 is an excellent composite photocatalyst under visible light.Photoluminescence (PL) spectra are a useful technique to survey the separation efficiency of the photogenerated charge carriers in a semiconductor, because PL emission mainly results from the recombination of free carriers.22 In general, the lower the PL intensity, the lower the recombination rate of photogenerated electron–hole pairs, and the higher the photocatalytic activity of semiconductor photocatalysts. Fig. 8 shows the PL spectra of the BiIO4/Bi2WO6 composites at different molar ratios at room temperature compared with that of pure Bi2WO6. It can be seen that 10% BiIO4/Bi2WO6 displays the lowest emission peaks, and thus possess the highest photocatalytic activity which is in good agreement with the result from photodegradation experiment.
According to the above experimental results, the enhanced photocatalytic activity of BiIO4/Bi2WO6 heterojunctions can be mainly attributed to the effective electron–hole separations at the interfaces of the two semiconductors. The photoinduced electron and hole could migrate to the surface to react with the adsorbed reactants, and the migration direction of the photogenerated charge carrier depends on the band edge positions of semiconductors. The band edge positions of the as-prepared semiconductors are theoretically predicted using electronegativity concept.27,28 The CB and VB potentials of the semi-conductor at the point of zero charge are calculated by the following equation:
| EVB = X − Ee + 0.5Eg | (2) |
| ECB = EVB − Eg | (3) |
Under visible light illumination (λ > 400 nm), BiIO4 and Bi2WO6 could be excited and induce the generation of photo-electrons and holes.22,23 According to their energy band position in Fig. 9, the conduction band gap potential of Bi2WO6 is more negative than that of BiIO4. Therefore, photo-generated electrons on the surface of Bi2WO6 could easily transfer into the conduction band of BiIO4 under the inducement action of the internal electric field, leaving holes on the Bi2WO6 valence band. Meanwhile, the photo-induced holes in the valance band of BiIO4 could be transferred to valance band of Bi2WO6. Thus, the photo-generated electrons and holes in the BiIO4 and Bi2WO6 could be separated effectively in the BiIO4/Bi2WO6 heterojunctions and the recombination of electron–hole pairs can be reduced, resulting in an enhanced photocatalytic activity. In addition, the coupling of two layered structure may also enhance the photoinduced electron–hole separation and transfer. The oxidation and reduction sites in photocatalytic reaction locate at the surface and edge position of two-dimensional layered structure, respectively. Thus, the photogenerated-holes only travel a very short distance (subnanometer) to reach the surface layer structure, and then were trapped by the hydroxyls in the layer gap. This rapid hole-trapping process allows more photogenerated-electrons more easily move to the edge of the layered structure, reducing the recombination probability of photogenerated-carriers. Thus, the presence of internal electric fields between [Bi2O2] and [WO6]/[IO3] are favorable for the efficient photoinduced electron–hole separation and transfer, which is also propitious to a high-photocatalytic efficiency of BiIO4/Bi2WO6 heterojunctions. However, due to the large band gap of BiIO4, the absorption of visible light will be decreased in BiIO4/Bi2WO6 composites with excess content of BiIO4, which will reduce the photocatalytic activity of the BiIO4/Bi2WO6 composite photocatalyst with more content of BiIO4.
Conclusions
In summary, we have successfully developed a novel BiIO4/Bi2WO6 heterojunction containing two layered structures by a facile hydrothermal method. The resulting composite catalysts were found to be composed of two kinds of nanosheets. The reasonable fabrication of BiIO4/Bi2WO6 heterojunctions is very beneficial for the separation and easy transfer of photo-generated electrons and holes at the intimate interface of heterojunctions, thus resulting in the enhanced visible-light-driven photocatalytic activity. The optimum photocatalytic activity of the 10% BiIO4/Bi2WO6 heterojunction for the degradation of RhB was almost 34.4 and 4.1 times higher than those of individual BiIO4 and Bi2WO6, respectively. This result indicated BiIO4/Bi2WO6 heterojunction is an excellent composite photocatalyst under visible light. Moreover, our research provided a new semiconductor, BiIO4 with an Aurivillius-related structure, which can be used for heterojunction construction and energy band structure design.Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (2652013052), and the National Natural Science Foundation of China under Grants 50590402, and 91022036, and the National Basic Research Project of China (2010CB630701, and 2011CB922204).Notes and references
- M. W. Kanan and D. G. Nocera, Science, 2008, 321, 1072–1075 CrossRef CAS PubMed.
- X. C. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. A. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- H. Tong, S. X. Ouyang, Y. P. Bi, N. Umezawa, M. Oshikiri and J. H. Ye, Adv. Mater., 2012, 24, 229–251 CrossRef CAS PubMed.
- A. L. Linsebigler, G. Lu and J. T. Yates, Chem. Rev., 1995, 95, 735–758 CrossRef CAS.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- A. Kubacka, M. Fernández-García and G. Colón, Chem. Rev., 2012, 112, 1555–1614 CrossRef CAS PubMed.
- J. Cao, B. Xu, B. Luo, H. Lin and S. Chen, Catal. Commun., 2011, 13, 63–68 CrossRef CAS PubMed.
- L. Q. Ye, J. Y. Liu, Z. Jiang, T. Y. Peng and L. Zan, Appl. Catal., B, 2013, 142, 1–7 Search PubMed.
- T. H. Yu, W. Y. Cheng, K. J. Chao and S. Y. Lu, Nanoscale, 2013, 5, 7356–7360 RSC.
- C. Xu, X. Wei, Y. Guo, H. Wu, Z. Ren, G. Xu, G. Shen and G. Han, Mater. Res. Bull., 2009, 44, 1635–1641 CrossRef CAS PubMed.
- H. Cheng, B. Huang, Y. Dai, X. Qin, X. Zhang, Z. Wang and M. Jiang, J. Solid State Chem., 2009, 182, 2274–2278 CrossRef CAS PubMed.
- M. Ge, Y. Li, L. Liu, Z. Zhou and W. Chen, J. Phys. Chem. C, 2011, 115, 5220–5225 CAS.
- Y. Fu, C. Chang, P. Chen, X. L. Chu and L. Y. Zhu, J. Hazard. Mater., 2013, 254, 185–192 CrossRef PubMed.
- S. Guo, X. F. Li, H. Q. Wang, F. Dong and Z. B. Wu, J. Colloid Interface Sci., 2012, 369, 373–380 CrossRef CAS PubMed.
- Y. Tian, L. D. Zhang and J. X. Zhang, J. Alloys Compd., 2012, 537, 24–28 CrossRef CAS PubMed.
- L. W. Zhang, Y. Man and Y. F. Zhu, ACS Catal., 2011, 1, 841–848 CrossRef CAS.
- H. Liu, J. Yuan, W. F. Shangguan and Y. Teraoka, J. Phys. Chem. C, 2008, 112, 8521–8523 CAS.
- W. Liu, S. F. Chen, H. Y. Zhang and X. L. Yu, J. Exp. Nanosci., 2011, 6, 102–120 CrossRef CAS.
- M. Shang, W. Z. Wang, L. Zhang, S. M. Sun, L. Wang and L. Zhou, J. Phys. Chem. C, 2009, 113, 14727–14731 CAS.
- D. Q. He, L. L. Wang, D. D. Xu, J. L. Zhai, D. J. Wang and T. F. Xie, ACS Appl. Mater. Interfaces, 2011, 3, 3167–3171 CAS.
- M. S. Gui, W. D. Zhang, Y. Q. Chang and Y. X. Yu, Chem. Eng. J., 2012, 197, 283–288 CrossRef CAS PubMed.
- H. Q. Li, Y. M. Cui and W. S. Hong, Appl. Surf. Sci., 2013, 264, 581–588 CrossRef CAS PubMed.
- Y. L. Tian, B. B. Chang, J. L. Lu, J. Fu, F. N. Xi and X. P. Dong, ACS Appl. Mater. Interfaces, 2013, 5, 7079–7085 CAS.
- S. D. Nguyen, J. Yeon, S. H. Kim and P. S. Halasyamani, J. Am. Chem. Soc., 2011, 133, 12422–12425 CrossRef CAS PubMed.
- X. Zhang, Z. H. Ai, F. L. Jia and L. Z. Zhang, J. Phys. Chem. C, 2008, 112, 747–753 CAS.
- H. W. Huang, Y. He, Z. S. Lin, L. Kang and Y. H. Zhang, J. Phys. Chem. C, 2013, 117, 22986–22994 CAS.
- D. Ke, T. Peng, L. Ma, P. Cai and P. Jiang, Appl. Catal., A, 2008, 350, 111–117 CrossRef CAS PubMed.
- L. Ren, J. Lei, J. B. Wang, M. Qiu and Y. Yu, Nanotechnology, 2009, 20, 405602 CrossRef PubMed.
- P. Madhusudan, J. R. Ran, J. Zhang, J. G. Yu and G. Liu, Appl. Catal., B, 2011, 110, 286–295 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: TEM images of pure Bi2WO6 samples. The first order kinetics curves for RhB photodegradation over BiIO4, Bi2WO6 and BiIO4/Bi2WO6 composites. See DOI: 10.1039/c3ra45891a |
| This journal is © The Royal Society of Chemistry 2014 |