Triphenylamine-containing microporous organic copolymers for hydrocarbons/water separation†
Yanqin Yangab,
Qiang Zhangab,
Suobo Zhang*a and
Shenghai Lia
aKey Laboratory of Polymer Ecomaterials, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China. E-mail: sbzhang@ciac.jl.cn; Fax: +86 431 85262117; Tel: +86 431 85262118
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 14th November 2013
Abstract
Two novel porous copolymers PBP-N-25 and PBP-N-50 were synthesized from triphenylamine and 4,4′-bis(chloromethyl)biphenyl using a combination of oxidative polymerization and Friedel–Crafts alkylation process promoted by anhydrous FeCl3. The polymers were predominantly microporous, with apparent BET surface areas of 1362 m2 g−1 for PBP-N-25 and 1338 m2 g−1 for PBP-N-50. PBP-N-25 and PBP-N-50 possessed benzene vapor uptakes of 94.1 wt% and 107.3 wt% and cyclohexane vapor uptakes of 95.3 wt% and 83.8 wt%, while the water vapor uptakes were only 1.7 wt% and 1.2 wt%, respectively. The large amount of phenyl and methylene linkers resulted in the polymers' hydrophobicity and affinity toward aromatic and aliphatic compounds. The benzene/water vapor selectivities of PBP-N-25 and PBP-N-50 were as high as 53.5 and 63.6. Monolithic polymer M-PBP-N-25 with an apparent BET surface area of 551 m2 g−1 was prepared, which exhibited a good performance for oil/water separation. Owing to its hydrophobic nature and low density, the monolith was floating on the surface of water before and after collecting all of the oil from water. After removing the monolith from the surface, oil and water separated instantly.
Introduction
Recent devastating cases of oil pollution caused by ships and offshore oil spills have intensively threatened all forms of life, especially human beings, which raises the urgent need for effective technologies for removing oils and organic pollutants from water and air.1 There are many sorbents in use for oil spill cleanup, including cotton fibers, organic clays, and activated carbons.2 There is still a need for novel and efficient absorbers with a considerable absorptivity, high selectivity and durability.Metal–organic frameworks (MOFs) are promising adsorbents for many guest molecules, such as H2, CO2 and organic vapor, but the poor water/moisture stability of most MOFs limits their application in oil spill cleanup.1c,3 Recently, microporous organic polymers (MOPs), which consist of purely organic structures via covalent bonds, have spurred scientific interest within the fast-developing field of sorbing materials.4 Depending on their synthesis methods and structures, MOPs have been classified into covalent organic frameworks (COFs),5 hyper-crosslinked polymers (HCPs),6 polymers with intrinsic microporosity (PIMs),7 conjugated microporous polymers (CMPs),8 and porous aromatic frameworks (PAFs).9 Compared with MOFs, these materials possess high thermal, chemical and water stabilities, low skeleton density as well as hydrophobic components in their structures. All these features make them outstanding candidates for oil spill cleanup.
Even in a small accident, there is usually several tons of oil spill.10 Therefore, scalability and pricing are important factors for synthesis of sorbents with a potential application. Among MOPs reported to date, HCPs are produced by the feasible strategies.6,11 Recently, we have synthesized a series of HCPs (named as PP-N-x, where “x” is the molar percent of triphenylamine) from triphenylamine and dichloro-p-xylene with anhydrous FeCl3 as catalyst.12 Our polymers exhibit cost-effective advantages, such as commercially available monomers, cheap catalyst and relatively mild reaction conditions. The nitrogen-containing structures make the networks electron-rich, resulting in their high CO2 uptake capacities.12 The polymers possess hydrophobic skeleton structures and large amounts of phenyl and methylene linkers, which may enhance affinity for both aromatic and aliphatic compounds.13 In a continuation of our previous study on PP-N-x, herein we report for the first time their strong oleophilicity towards hydrocarbons. For comparison purposes, we also synthesized analogues of PP-N-x (named as PBP-N-x) from copolymerization of triphenylamine and 4,4′-bis(chloromethyl)biphenyl.
Experimental part
Materials
Triphenylamine (TPA) and dichloro-p-xylene (DCX) were purchased from Alfa Aesar. Anhydrous FeCl3 and 4,4′-bis(chloromethyl)biphenyl (BCMBP) were obtained from Aladdin. 1,2-Dichloroethane (DCE) was dried over CaH2 prior to use. Other chemicals were used as received.Synthesis of triphenylamine-containing porous copolymers
PP-N-25 and PP-N-50 were synthesized according to our previous study.12 The analogues of PP-N-25 and PP-N-50, i.e. PBP-N-25 and PBP-N-50, were produced by following an analogous procedure. A continuous monolithic network of PBP-N-25, denoted as M-PBP-N-25, was also prepared. The representative preparations for PBP-N-25 and M-PBP-N-25 are given in detail as follows.Characterization
The FT-IR spectra were obtained using a Bruker Vertex 70 spectrometer at a nominal resolution of 2 cm−1. Solid-state NMR spectra were obtained on a Chemagnetics CMS-400 spectrometer. Elemental analysis was performed on an Elementar Analysensysteme GmbH (vario EL cube). TGA was performed on a PU 4K (Rigaku) at a heating rate of 10 °C min−1 with N2 as carrier gas. Size and morphology of the samples were observed under a field-emission scanning electron microscope (HitachiS-4800). SEM observations were carried out using a HitachiS-4800 microscope (Hitachi Ltd., Japan) at an accelerating voltage of 6.0 kV and equipped with a Horiba energy dispersive X-ray spectrometer. Volumetric gas and vapor adsorption was performed on an Autosorb iQ2 instrument (Quantachrome). Nitrogen adsorption isotherms at 77 K were measured in liquid-nitrogen baths. For the vapor adsorption process, P and Po are the vapor pressure of the adsorbate in adsorption measurements and the saturated vapor pressure of the adsorbate, respectively, and P/Po denotes the relative pressure of the adsorbate.Liquid uptake measurement
Before each absorption test, the polymers were evacuated at 100 °C for 10 h. For powdery polymers, the weighed quantities (0.1500 g) of samples were immersed in various organic solvents at room temperature to reach the absorption equilibrium. The wet samples were separated from the organic solvents by filtration and weight measurements were performed quickly to avoid evaporation of absorbed solvents. For monolithic polymer, the wet sample was taken off after some predetermined intervals, gently dried with paper tissue to remove adhering solvent, and weight measurements were done quickly to avoid evaporation of absorbed solvent. The liquid uptake (LU) of the samples was calculated using the following equation:| LU = (Wwet − Wdry)/Wdry × 100% |
Results and discussion
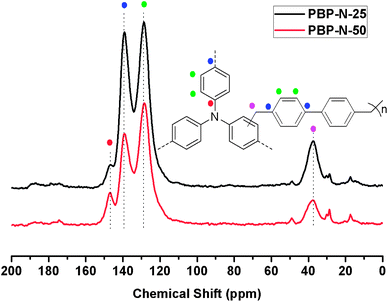
As shown in Scheme 1, the preparation process was carried out by a one-step copolymerization in DCE with anhydrous FeCl3 as catalyst. The Friedel–Crafts alkylation process between the two monomers arising from benzyl chloride and oxidative self-polymerization of TPA concurred to produce the insoluble polymers (named as PBP-N-x, where “x” is the molar percent of triphenylamine). With the different activities of TPA, its oligomer and BCMBP towards Friedel–Crafts alkylation, it was unlikely that both monomers were incorporated at the same rate. However, because of the insolubility of the polymers formed, characterizing the distribution of monomers was very difficult.We synthesized both powdery and monolithic states of polymers by adjusting the reactant concentration. The polymers in a fine powdery state were produced when the solid content was about 2.7%, while continuous monolithic polymers were formed in the presence of a limited amount of solvent (the solid content was around 20%). The porous polymers were characterized from their FT-IR spectra (Fig. 1). Saturated N–C stretching bands at 1320 cm−1 meant the successful incorporation of TPA into the polymers. The band at 2915 cm−1 is ascribed to C–H stretching vibrations originating from –CH2– of BCMBP. The characteristic bands at 1265 and 679 cm−1 resulting from the wagging and stretching vibration of the CH2–Cl group disappeared, which implied the total consumption of BCMBP. PBP-N-25 and M-PBP-25 possessed similar FT-IR spectra, which indicated that they exhibited almost identical chemical structures even though they were in different states. The structure of the networks has been characterized at the molecular level by 13C solid-state NMR and the signal assignments for the spectra are displayed in Fig. 2. The 13C solid-state NMR confirmed the presence of sp2 carbons from the TPA and BCMBP as well as sp3 carbons from methylene linkers. Furthermore, elemental combustion analysis was used to test the chemical identity of the polymers (Table S1†). For M-PBP-N-25, the observed C/N/H value was consistent with the theoretical one, confirming the successful synthesis of the polymer. However, for PBP-N-25 and PBP-N-50, there was a deviation between the two values. Similar to other powdery MOPs, this was due to the incomplete combustion and adsorption of gases and moisture of the samples.
The morphologies of the polymers were investigated by field-emission scanning electron microscopy (SEM) (Fig. 3). PBP-N-25 and PBP-N-50 consisted of irregular particles, existing some obvious macropores (about 100 to 500 nm), whereas M-PBP-N-25 was composed by regular fused spheres with diameters of 100 nm. We used thermogravimetric analysis (TGA) to investigate the thermal stability of the polymers (Fig. S1†). The polymers exhibited a second-step degradation process. The first weight loss from 150 °C to 220 °C was ascribed to decomposition of methylene linkers, whose thermal stability was weak compared with aromatic rings.
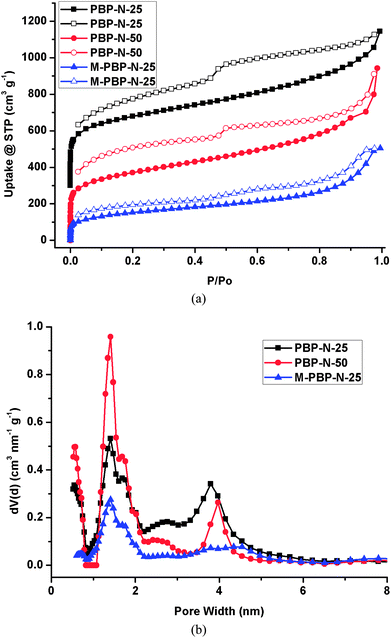
The pore structures of the polymers were evaluated by nitrogen sorption isotherms measured at 77 K (Fig. 4). All polymers showed type-I isotherms, which are typical for microporous materials. Significant hysteresis was observed in the desorption branch of the isotherms for all samples, indicating that their porous skeletons were highly swellable.14 Table 1 summarizes the textural parameters calculated from these isotherms. The apparent Brunauer–Emmett–Teller (BET) surface areas of PBP-N-25 and PBP-N-50 were 1362 and 1338 m2 g−1, slightly higher than that of their analogues (1257 m2 g−1 for PP-N-25 and 1141 m2 g−1 for PP-N-50).12 However, the total pore volumes of PBP-N-25 (1.307 cm3 g−1) and PBP-N-50 (1.458 cm3 g−1) were much higher than that of PP-N-25 (1.056 cm3 g−1) and PP-N-50 (1.084 cm3 g−1). M-PBP-N-25 exhibited an apparent BET surface area of 551 m2 g−1, which was much lower than that of PBP-N-25. An explanation for the small N2 uptake of M-PBP-N-25 was the slow mass transport of N2 into the strictly microporous M-PBP-N-25 monolith at 77 K.15 To confirm that mass transport had an important role in M-PBP-N-25's N2 sorption, we pulverized the monolith and remeasured the N2 sorption isotherm (Fig. S2a†). As expected, the total pore volume increased from 0.759 cm3 g−1 to 1.411 cm3 g−1. Nevertheless, the apparent BET surface area merely increased to 769 m2 g−1, still less than PBP-N-25. Therefore, slow mass transport was not the major cause of M-PBP-N-25’s low surface area. An additional explanation was the high solid content (around 20%) during the preparation of M-PBP-N-25, which resulted in the fast deposition of polymer, hindering the further crosslinking of the residual unreacted groups. The pore size calculated from nonlinear density functional theory (NLDFT) showed that PBP-N-25 and PBP-N-50 possess both micropores and mesopores centered at around 1.5 and 4 nm (Fig. 4b). M-PBP-N-25 possessed only micropore with a diameter of 1.5 nm, while pulverized M-PBP-N-25 also had several pores in the mesoporous range (Fig. S2b†).
| Sample | SBETa (m2 g−1) | SLangb (m2 g−1) | Smicroc (m2 g−1) | Vmicroc (cm3 g−1) | VTold (cm3 g−1) |
|---|---|---|---|---|---|
| a Apparent surface area calculated over the relative pressure range P/Po = 0.1–0.2 using the BET method.b Apparent surface area calculated over the relative pressure range P/Po = 0.1–0.2 using the Langmuir method.c Microporous BET surface area and micropore volume calculated over the relative pressure range P/Po = 0.2–0.5 using t-plot method micropore analysis.d Total pore volume (P/Po = 0.975).e Ref. 12. | |||||
| PBP-N-25 | 1362 | 1568 | 668 | 0.287 | 1.307 |
| PBP-N-50 | 1338 | 1541 | 683 | 0.291 | 1.458 |
| PP-N-25e | 1257 | 1402 | 831 | 0.352 | 1.056 |
| PP-N-50e | 1141 | 1275 | 579 | 0.248 | 1.084 |
| M-PBP-N-25 | 551 | 701 | 223 | 0.095 | 0.759 |
| M-PBP-N-25 (pulverized) | 769 | 1039 | 310 | 0.135 | 1.411 |
The adsorption isotherms of the polymers for water vapor were typical type III sorption (Fig. S3†), indicating the hydrophobic nature of their skeletons. The isotherms of PP-N-25 and PP-N-50 had an abrupt increase above the relative humidity of 60%, due to water vapor condensation on the exterior surface.16 However, no similar observation was found for PBP-N-25 and PBP-N-50's isotherms. At P/Po = 0.8, the water vapor uptakes of PBP-N-25 and PBP-N-50 were only 1.7 wt% and 1.2 wt%, while for PP-N-25 and PP-N-50, these values reached 19.8 wt% and 9.5 wt%, respectively. Relatively high hydrophobicity of PBP-N-x stemmed from the large amount of strongly hydrophobic biphenyl units in their structures. In order to confirm their hydrophobic surface properties, we also measured their water contact angle (CA). It was observed that PBP-N-25, PBP-N-50, PP-N-25 and PP-N-50 exhibited water CA of 145°, 142°, 132° and 134°, respectively (Fig. S4†), indicating that their surfaces are strongly hydrophobic.
We used benzene, cyclohexane, and n-hexane, which embody the components of oil, as the probe molecules to evaluate the polymers' performances in sorbing petroleum. The adsorption isotherms for benzene, cyclohexane, and n-hexane vapors were displayed in Fig. 5. Different from water vapor adsorption, four polymers exhibited a rapid rise of the C6 hydrocarbon uptakes at the low relative pressure (P/Po < 0.1), indicating the skeletons have a strong affinity towards these molecules. Due to the strong π–π interactions between benzene and the phenyl groups, PBP-N-x possessed high benzene vapor capture capacities (94.1 wt% for PBP-N-25 and 107.3 wt% for PBP-N-50) comparable to that of tetraphenyladamantane-based microporous polyimide (PI-ADPM).17 It was interesting to observe that the presence of methylene linkers resulted in PBP-N-x possessing significantly higher uptake of aliphatic hydrocarbon vapors than the wholly aromatic porous polymers. For instance, the adsorbed amount of cyclohexane for PBP-N-25 and PBP-N-50 were as high as 95.3 wt% and 83.8 wt%, respectively, whereas the totally aromatic framework PAF-2 was only 0.7 wt%.18 These values are also higher or comparable to those of PI-ADPM17 and PCPF-1 (ref. 16) reported recently exhibiting exceptional uptake capacity of saturated hydrocarbons. Compared with PBP-N-x, PP-N-x possessed lower organic vapor uptake capacities. These originated from the weaker hydrophobicity and smaller pore volumes of PP-N-x. The adsorption selectivities for binary mixtures were calculated from initial slopes of pure-component adsorption isotherms. As shown in Table 2, PBP-N-x exhibited excellent organic-vapor/water selectivities. For example, the benzene/water selectivities of PBP-N-25 and PBP-N-50 were as high as 53.5 and 63.6, outperforming the recently reported microporous polyimide materials of MPI-1 (28), MPI-2 (13) and MPI-3 (14).19
 | ||
| Fig. 5 Adsorption isotherms of the polymers for benzene (a), cyclohexane (b), and hexane (c) vapor at 298 K; (d) liquid uptake of the polymers at room temperature. | ||
| Sample | Vapor uptake at P/Po = 0.8 (wt%) | Vapor selectivity | Liquid uptake (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H2O | C6H6 | c-C6H12 | n-C6H14 | C6H6/H2O | c-C6H12/H2O | n-C6H14/H2O | C6H6 | c-C6H12 | n-C6H14 | |
| PBP-N-25 | 1.7 | 94.1 | 95.3 | 65.3 | 53.5 | 23.9 | 26.5 | 766.6 | 568.9 | 399.3 |
| PBP-N-50 | 1.2 | 107.3 | 83.8 | 72.8 | 63.6 | 29.3 | 20.4 | 832.4 | 781.3 | 608.0 |
| PP-N-25 | 19.8 | 45.0 | 50.7 | 42.3 | 11.2 | 5.7 | 3.7 | 424.6 | 347.6 | 232.6 |
| PP-N-50 | 9.5 | 49.8 | 49.5 | 46.5 | 15.3 | 10.9 | 6.7 | 807.8 | 744.0 | 626.4 |
The excellent vapor adsorption performance of the polymers motivated us to further investigate their potential for absorption of liquid-phase oil. Due to the strong capillary force in the nanometer-sized pores, the polymers reached absorption equilibrium within 30 seconds. Surprisingly, PBP-N-50 and PP-N-50 possessed similar liquid uptake (LU) capacities, higher than those of PBP-N-25 and PP-N-25 (Table 2). This is due to the fact that liquid-phase uptakes are the synergistic effect of porosity and swellability.20 The high porosity provides a large interface, and the swellability boosts the absorption capacity. As shown in Fig. 5d and Table 1, PBP-N-50 and PP-N-50 exhibited a larger pore volume than their analogues PBP-N-25 and PP-N-25. Furthermore, compared with PBP-N-25 and PP-N-25, PBP-N-50 and PP-N-50 possessed low content of DCX or BCMBP which was used as crosslinking agent, leading to the polymers' high swelling ability. As expected, they absorb almost no liquid-phase water due to their hydrophobicity and oleophilicity. As a result, oil or non-polar organic solvents can be easily removed from water by the polymers without adsorption of water.
As spilt oil usually spreads on the seawater, it is impractical to achieve the oil/water separation by filtration the mixture of seawater and powdery sorbents. If some stick-shaped sorbing material was used, the oil can be easily separated from water simply by taking out the saturated stick from the water without further processing. Therefore, taking cyclohexane as an example, we measured in detail the M-PBP-N-25's liquid-phase solvent sorption behavior (Fig. 6). Fig. 6a showed the absorption curve of M-PBP-N-25 with a particular size (diameter (d) = 0.4940 cm, length (L) = 1.4257 cm) as a function of absorption time. Three absorption stages may be distinguished in the curve: slow growth part, rapid growth part and saturation range or plateau. At the beginning of absorption, the solvent mainly diffused into the stick from the bottom surfaces, which were looser than the side surface (Fig. 6c1). The solvent molecules produced solvation with the hydrophobic structure, then swelled the material and remained in the newly enlarged pores. As time went on, both ends of the stick swelled obviously, and the uniform stick became a dumbbell shape (Fig. 6c2). At the rapid growth part, diffusion also widely occurred at the swollen section (Fig. 6c3–4). After 130 min, the stick swelled fully (d = 0.8840 mm, L = 2.1730 mm), and the absorption process slowed down. The absorptivity process reached the saturation range at 140 min. Due to this kind of sorption mechanism, the absorption rate of a stick sample can be enhanced with increasing the d/L ratio. Compared with its powdery analogue PBP-N-25, the sorption of monolithic M-PBP-N-25 was very slow. A rapid solvent sorption of a porous material always depends on its interconnected pores (especially the macropores) and easy swelling structures.21 Unlike the powdery sample, the monolithic sample is predominantly microporous without obvious inter-particle macropores (Fig. 3). When solvent comes in contact with M-PBP-N-25, the bulky cyclohexane molecules can only enter the surface pores. The non-polar solvent molecules and hydrophobic structure produce solvation, which expands the molecular chain and produces new free volume and pores. Then more solvent molecules enter into the swollen materials and remain in the newly emerged pores. Because of the hyper-crosslinked skeleton, the polymer's swelling process is slow, resulting in a slow sorption process. The saturation absorption capacity of M-PBP-N-25 was 348.4 wt%, lower than that of PBP-N-25 (568.9 wt%), because of the smaller pore volume of the former (0.759 cm3 g−1 vs. 1.307 cm3 g−1). The desorption process of the stick was carried out under vacuum and room temperature. The curve had a rapid decline within the first 4 min, during which time about 83% cyclohexane desorbed. At 10 min, 95% solvent was desorbed. As a result, the sorbents and solvents or oil could be easily recovered via a low-pressure distillation.
When a small piece of M-PBP-N-25 was placed on the surface of a stratified cyclohexane–water mixture in a glass beaker, the red-colored cyclohexane can be absorbed by the monolith within several minutes (Fig. 7). Such fast absorption kinetics should be attributed to the large d/L ratio of the monolith, strong oleophilic nature of the polymer combined with its microporous features where absorbing of cyclohexane by capillary action of its surface nanometer pores may occur as well.22 Owing to its hydrophobic nature and low density, the monolith was floating on the surface of water before and after collecting all of the oil from water. After removing the monolith from the beaker, oil and water were separated instantly.
 | ||
| Fig. 7 Snapshots showing the adsorption of red-colored cyclohexane film (dyed with red oil) distributed on a water bath by a piece of M-PBP-N-25. | ||
Conclusion
We synthesized two kinds of microporous polymers PBP-N-25 and PBP-N-50, which had apparent BET surface areas of 1362 m2 g−1 and 1338 m2 g−1, respectively. Two polymers exhibited similar uptakes for vapor-phase water, benzene, cyclohexane, and n-hexane. However, due to PBP-N-50’s looser and highly swellable structure and large pore volume, it exhibited higher liquid-phase C6 hydrocarbon uptake capacities than those of PBP-N-25. Monolithic network M-PBP-N-25, with a similar chemical structure of its powdery counterpart PBP-N-25, was also prepared. M-PBP-N-25 possessed an apparent BET surface area of 551 m2 g−1, while pulverized M-PBP-N-25 had an apparent BET surface area of 769 m2 g−1, lower than that of PBP-N-25. This was due to the high solid content (around 20%) of M-PBP-N-25 during its preparation process which resulted in the fast deposition of polymer, hindering the further crosslinking of the residual unreacted groups. Owing to its hydrophobic nature and low density, the monolith was floating on the surface of water before and after collecting of oil from water. After removing the monolith from the beaker, oil and water were separated instantly. The high oleophilicity and hydrophobicity as well as easy operability give M-PBP-N-25 the potential for application in oil spill cleanup.Acknowledgements
This research was financially supported by the National Basic Research Program of China (no. 2012CB932802), the National Science Foundation of China (no. 51133008, 51021003, 21074133), the National High Technology Research and Development Program of China (no. 2012AA03A601), and the Development of Scientific and Technological Project of the Jilin Province (no. 20100322).Notes and references
-
(a) A. Godduhn and L. K. Duffy, Environ. Sci. Policy, 2003, 6, 341 CrossRef CAS
; (b) Y. Zhang, S. Wei, F. Liu, Y. Du, S. Liu, Y. Ji, T. Yokoi, T. Tatsumi and F.-S. Xiao, Nano Today, 2009, 4, 135–142 CrossRef CAS PubMed
; (c) X. Wang, J. Liu, J. M. Bonefont, D.-Q. Yuan, P. K. Thallapally and S. Ma, Chem. Commun., 2013, 49, 1533 RSC
; (d) L.-P. Xu, J. Zhao, B. Su, X. Liu, J. Peng, Y. Liu, H. Liu, G. Yang, L. Jiang, Y. Wen, X. Zhang and S. Wang, Adv. Mater., 2013, 25, 606 CrossRef CAS PubMed
.
-
(a) C. Yang, U. Kaipa, Q. Z. Mather, X. Wang, V. Nesterov, A. F. Venero and M. A. Omary, J. Am. Chem. Soc., 2011, 133, 18094 CrossRef CAS PubMed
; (b) J. Drelich, J. Hupka and B. Gutkowski, Stud. Environ. Sci., 1988, 34, 207 CAS
.
-
(a) H. Ren, T. Ben, F. Sun, M. Guo, X. Jing, H. Ma, K. Cai, S. Qiu and G. Zhu, J. Mater. Chem., 2011, 21, 10348 RSC
; (b) Z. Zhang, W.-Y. Gao, L. Wojtas, S. Ma, M. Eddaoudi and M. J. Zaworotko, Angew. Chem., Int. Ed., 2012, 51, 9330 CrossRef CAS PubMed
.
-
(a) N. B. McKeown and P. M. Budd, Chem. Soc. Rev., 2006, 35, 675 RSC
; (b) R. E. Morris and P. S. Wheatley, Angew. Chem., Int. Ed., 2008, 47, 4966 CrossRef CAS PubMed
; (c) J. X. Jiang, C. Wang, A. Laybourn, T. Hasell, R. Clowes, Y. Z. Khimyak, J. Xiao, S. J. Higgins, D. J. Adams and A. I. Cooper, Angew. Chem., Int. Ed., 2011, 50, 1072 CrossRef CAS PubMed
; (d) R. Dawson, D. J. Adams and A. I. Cooper, Chem. Sci., 2011, 2, 1173 RSC
; (e) M. W. Schneider, I. M. Oppel and M. Mastalerz, Chem.–Eur. J., 2012, 18, 4156 CrossRef CAS PubMed
.
-
(a) H. M. El-Kaderi, J. R. Hunt, J. L. Mendoza-Cortes, A. P. Cote, R. E. Taylor, M. O'Keeffe and O. M. Yaghi, Science, 2007, 316, 268 CrossRef CAS PubMed
; (b) E. L. Spitler and W. R. Dichtel, Nat. Chem., 2010, 2, 672 CrossRef CAS PubMed
; (c) J. W. Colson, A. R. Woll, A. Mukherjee, M. P. Levendorf, E. L. Spitler, V. B. Shields, M. G. Spencer, J. Park and W. R. Dichtel, Science, 2011, 332, 228 CrossRef CAS PubMed
.
-
(a) M. P. Tsyurupa and V. A. Davankov, React. Funct. Polym., 2006, 66, 768 CrossRef CAS PubMed
; (b) J. Germain, J. Hradil, J. M. J. Frechet and F. Svec, Chem. Mater., 2006, 18, 4430 CrossRef CAS
.
-
(a) P. M. Budd, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib and C. E. Tattershall, Chem. Commun., 2004, 230 RSC
; (b) N. Du, H. B. Park, G. P. Robertson, M. M. Dal-Cin, T. Visser, L. Scoles and M. D. Guiver, Nat. Mater., 2011, 10, 372 CrossRef CAS PubMed
; (c) J. Weber, N. Du and M. D. Guiver, Macromolecules, 2011, 44, 1763 CrossRef CAS
.
-
(a) J. X. Jiang, F. Su, A. Trewin, C. D. Wood, H. Niu, J. T. A. Jones, Y. Z. Khimyak and A. I. Cooper, J. Am. Chem. Soc., 2008, 130, 7710 CrossRef CAS PubMed
; (b) R. Dawson, D. J. Adams and A. I. Cooper, Chem. Sci., 2011, 2, 1173 RSC
.
-
(a) T. Ben, H. Ren, S. Q. Ma, D. P. Cao, J. H. Lan, X. F. Jing, W. C. Wang, J. Xu, F. Deng, J. M. Simmons, S. L. Qiu and G. S. Zhu, Angew. Chem., Int. Ed., 2009, 48, 9457 CrossRef CAS PubMed
; (b) H. Ren, T. Ben, E. S. Wang, X. F. Jing, M. Xue, B. B. Liu, Y. Cui, S. L. Qiu and G. S. Zhu, Chem. Commun., 2010, 46, 291 RSC
.
- C. A. Kontovas, H. N. Psaraftis and N. P. Ventikos, Mar. Pollut. Bull., 2010, 60, 1455 CrossRef CAS PubMed
.
-
(a) B. Li, R. Gong, W. Wang, X. Huang, W. Zhang, H. Li, C. Huand and B. Tan, Macromolecules, 2011, 44, 2410 CrossRef CAS
; (b) M. G. Schwab, A. Lennert, J. Pahnke, G. Jonschker, M. Koch, I. Senkovska, M. Rehahn and S. Kaskel, J. Mater. Chem., 2011, 21, 2131 RSC
; (c) Y. Luo, B. Li, W. Wang, K. Wu and B. Tan, Adv. Mater., 2012, 24, 5702 CrossRef
.
- Y. Yang, Q. Zhang, S. Zhang and S. Li, Polymer, 2013, 54, 5698 CrossRef CAS PubMed
.
- C. Shen, Y. Bao and Z. Wang, Chem. Commun., 2013, 49, 3321 RSC
.
- J. Weber, M. Antonietti and A. Thomas, Macromolecules, 2008, 41, 2880 CrossRef CAS
.
- P. Mohanty, L. D. Kull and K. Landskron, Nat. Commun., 2011, 2, 401 CrossRef PubMed
.
- X.-S. Wang, J. Liu, J. M. Bonefont, D.-Q. Yuan, P. K. Thallapally and S. Ma, Chem. Commun., 2013, 49, 1533 RSC
.
- C. Shen, Y. Bao and Z. Wang, Chem. Commun., 2013, 49, 3321 RSC
.
- H. Ren, T. Ben, E. Wang, X. Jing, M. Xue, B. Liu, Y. Cui, S. Qiu and G. Zhu, Chem. Commun., 2010, 46, 291 RSC
.
- G. Li and Z. Wang, Macromolecules, 2013, 46, 3058 CrossRef CAS
.
- X. Liu, Y. Xu, Z. Guo, A. Nagai and D. Jiang, Chem. Commun., 2013, 49, 3233 RSC
.
- H. Yan, X. Liu, J. Zou, T. Gu, W. Chai and H. Li, ACS Appl. Mater. Interfaces, 2013, 5, 7737 Search PubMed
.
- A. Li, H.-X. Sun, D.-Z. Tan, W.-J. Fan, S.-H. Wen, X.-J. Qing, G.-X. Li, S.-Y. Li and W.-Q. Deng, Energy Environ. Sci., 2011, 4, 2062 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra44919j |
| This journal is © The Royal Society of Chemistry 2014 |