Polypyrrole nanotubes: mechanism of formation
Abstract
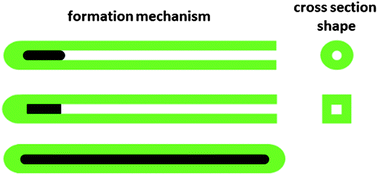
This article presents a contribution to better understanding of the processes which take place during the synthesis of polypyrrole nanotubes using a structure-guiding agent, methyl orange. Polypyrrole was prepared by oxidation of pyrrole monomer with iron(III) chloride. In the presence of methyl orange, the formation of nanotubes was observed instead of the globular morphology. Two reaction schemes with reversed additions of oxidant and monomer have been tested and they show remarkable influence on the produced morphology. Nanotubes with circular or rectangular profiles and diameters from tens to hundreds of nanometres have been obtained. FTIR and Raman spectra were used to assess the molecular structure of polypyrrole and detect residual methyl orange in the samples. The conductivity of nanotubes compressed into pellets was as high as 68 S cm−1. The mechanism of nanotubular formation starting at the nucleus produced with the participation of organic dye is proposed. The growth of a nanotube, however, proceeds in the absence of a template. An alternative mechanism for the formation of nanotubes, the coating of solid templates with a polypyrrole overlayer, is also discussed.

 Please wait while we load your content...
Please wait while we load your content...