DOI:
10.1039/C3RA45785K
(Paper)
RSC Adv., 2014,
4, 1163-1167
BODIPY dye possessing solid-state red fluorescence and green metallic luster properties in both crystalline and amorphous states†
Received
12th October 2013
, Accepted 6th November 2013
First published on
14th November 2013
Abstract
Carbazole-BODIPY dyes YHO-2 and YHO-3, which have diphenylamino-carbazole moiety as an electron-donating group at the 8-position on the BODIPY core, and heptanoic acid for YHO-2 and ethyl heptanoate for YHO-3 on the carbazole ring, were designed and synthesized and their photophysical properties in solution and in the solid state were investigated. Absorption and fluorescence properties of YHO-2 and YHO-3 are similar in solution, and both the dyes exhibited moderate fluorescence quantum yields. However, as-recrystallized dye YHO-3 exhibits solid-state red fluorescence but as-recrystallized dye YHO-2 exhibits feeble solid-state fluorescence properties. Interestingly, the dye YHO-3 possesses green metallic luster properties in both crystalline and amorphous states. Moreover, it was found that by grinding the as-recrystallized dye YHO-3, the disappearance of the metallic luster property and the blue-shift of the fluorescence maximum were observed in the ground solid. On the basis of X-ray powder diffraction (XRD) and differential scanning calorimetry (DSC), the solid-state photophysical properties of the carbazole-BODIPY dyes are discussed.
Introduction
Boron dipyrromethene (BODIPY) dyes are attractive materials not only from the viewpoint of fundamental research fields of photochemistry and photophysics, but also for optoelectronic devices such as organic light-emitting diodes (OLED), organic photovoltaics (OPV), and dye-sensitized solar cells (DSSC), because of their large photoabsorption coefficient in the visible and red/near-IR (NIR) region of the solar spectrum, strong fluorescence properties, and electrochemical modifications through the introduction of electron-donating and electron-accepting groups onto the BODIPY core, as well as their high chemical stabilities and photostability, and high solubility in organic solvents.1–8 A number of BODIPY dyes have been designed and developed and their photophysical and electrochemical properties were investigated. In particular, the solid-state photophysical properties of BODIPY dyes are of scientific and practical interest, so that much research was conducted on the relationship between the solid-state photophysical properties and the molecular packing structures.9–12 For example, Akkaya et al. have reported the solid-state red emissive BODIPY dyes with bulky substituents as spacers; introduction of bulky tert-butyl substituents on the meso-phenyl groups results in more spaced packing in the solid-state, leading to highly luminescent powders and films.9
Recently, on the other hand, we have designed and synthesized carbazole-BODIPY dyes YHO-2 (ref. 13) and YHO-3, which have diphenylamino-carbazole moiety as an electron-donating group at the 8-position on the BODIPY core, and heptanoic acid for YHO-2 and ethyl heptanoate for YHO-3 on the carbazole ring (Scheme 1), and investigated their photophysical properties in solution and in the solid state. We found that YHO-3 possesses not only red fluorescence properties in the crystalline state but also green metallic luster properties in both crystalline and amorphous states. Some research on organic dyes with metallic luster properties in the crystalline state have been reported,14–18 but to our knowledge, there is no research on the BODIPY dye possessing metallic luster properties not only in the crystalline state but also in the amorphous state. The metallic luster properties of organic dyes has received considerable attention from chemists, physicists, and engineers because of the enormous scientific interest in the photochemistry and photophysics and interesting functional materials for next generation optoelectronic devices, as well as organic metallic luster pigments.14–18 Moreover, it was revealed that by grinding the as-recrystallized dye YHO-3, the disappearance of the metallic luster property and the blue-shift of the fluorescence maximum are observed in the ground solid. However, the dye YHO-2 did not exhibit solid-state fluorescence and metallic luster properties. Herein, on the basis of X-ray powder diffraction (XRD) and differential scanning calorimetry (DSC), we discuss the solid-state photophysical properties of the carbazole-BODIPY dyes YHO-2 and YHO-3.
 |
| | Scheme 1 Synthesis of BODIPY dyes YHO-2 and YHO-3. | |
Results and discussion
Synthesis of YHO-2 and YHO-3
The carbazole-BODIPY dyes YHO-2 (ref. 13) and YHO-3 were prepared by condensation of compound 1 (ref. 13) or 2 (ref. 19) with 2-ethyl-1,3-dimethylpyrrole with added TFA as catalyst followed by oxidation with DDQ and treatment with BF3–OEt2 (Scheme 1).
Spectroscopic properties of YHO-2 and YHO-3 in solution
The absorption and fluorescence spectra of YHO-2 and YHO-3 in 1,4-dioxane are shown in Fig. 1. The two dyes show strong absorption peak at 524 nm, which is assigned to the S0 → S1 transition of the BODIPY core. The molar extinction coefficient (ε) at the absorption peak wavelength is 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 M−1 cm−1 for YHO-2 and 77
600 M−1 cm−1 for YHO-2 and 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1 cm−1 for YHO-3, respectively. The absorption peak at 371 nm (ε = 39
800 M−1 cm−1 for YHO-3, respectively. The absorption peak at 371 nm (ε = 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 45
000 and 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 M−1 cm−1 for YHO-2 and YHO-3, respectively) which can be assigned to the intramolecular charge transfer (ICT) band due to phenylcarbazole-diphenylamine moiety, was also observed.13 The absorption band at around 320 nm is attributed to the local π → π* transition.20 The fluorescence maximum appears at 535 nm for YHO-2 and 536 nm for YHO-3, respectively, which occurs from the BODIPY locally excited (LE) state. The fluorescence quantum yields (Φ) of YHO-2 and YHO-3 are 0.62 and 0.70, respectively. The differences of ε and Φ values between YHO-2 and YHO-3 may be attributed to the difference in interaction between the dye and solvent, that is, the difference in affinity of dye for solvent due to carboxylic acid of YHO-2 and carboxylic acid ester for YHO-3. However, the absorption and fluorescence spectra of YHO-2 and YHO-3 resemble each other very closely, that is, this result indicates that the photophysical properties of dye themselves are similar between YHO-2 and YHO-3.
300 M−1 cm−1 for YHO-2 and YHO-3, respectively) which can be assigned to the intramolecular charge transfer (ICT) band due to phenylcarbazole-diphenylamine moiety, was also observed.13 The absorption band at around 320 nm is attributed to the local π → π* transition.20 The fluorescence maximum appears at 535 nm for YHO-2 and 536 nm for YHO-3, respectively, which occurs from the BODIPY locally excited (LE) state. The fluorescence quantum yields (Φ) of YHO-2 and YHO-3 are 0.62 and 0.70, respectively. The differences of ε and Φ values between YHO-2 and YHO-3 may be attributed to the difference in interaction between the dye and solvent, that is, the difference in affinity of dye for solvent due to carboxylic acid of YHO-2 and carboxylic acid ester for YHO-3. However, the absorption and fluorescence spectra of YHO-2 and YHO-3 resemble each other very closely, that is, this result indicates that the photophysical properties of dye themselves are similar between YHO-2 and YHO-3.
 |
| | Fig. 1 Absorption (—) and fluorescence (⋯) spectra (λex = 509 nm) of YHO-2 and YHO-3 in 1,4-dioxane. | |
Photophysical properties of YHO-2 and YHO-3 in the solid-state
Fig. 2 shows the colors of the dyes YHO-2 and YHO-3, which are recrystallized from a mixed solvent of ethyl acetate–n-hexane. The color of as-recrystallized dye YHO-2 is reddish brown. On the other hand, the as-recrystallized dye YHO-3 possesses green metallic luster. The metallic luster property seems to depend on the terminal COOEt group in YHO-3, because YHO-2 bearing the COOH group is inactive in the metallic luster property. It is suggested that the hydrogen bonding formation due to the COOH group in YHO-2 may disturb the formation of the metallic luster. The as-recrystallized dye YHO-3 exhibited solid-state red fluorescence. Moreover, we found that by grinding the as-recrystallized dye YHO-3, the disappearance of metallic luster property were observed in the ground solid, although the ground solid keeps the solid-state red fluorescence property. On the other hand, the dye YHO-2 before and after grinding of the as-recrystallized dye exhibited very feeble solid-state fluorescence properties. Thus, in order to investigate the solid-state photophysical properties of YHO-2 and YHO-3, we have measured the solid-state UV-Vis diffuse reflection–absorption spectra (Fig. 3), and the fluorescence excitation and emission spectra of the solids (Fig. 4). The as-recrystallized dye YHO-3 shows the broadened absorption band in a visible region of 300 to 800 nm and the valley of absorption at around 560 nm. On the other hand, for the ground solid the absorption spectrum exhibits a plateau in a visible region of 300 to 570 nm and the valley of absorption at around 560 nm disappears. The corresponding fluorescence maximum (λflmax) of the as-recrystallized dye occurs at around 713 nm, which is significantly red-shifted by 177 nm compared with that in 1,4-dioxane. The λflmax of ground solid occurs at around 650 nm, which is blue-shifted by 63 nm compared with that of the as-recrystallized dye. The solid-state fluorescence quantum yield of YHO-3 was 0.06 before and after grinding of the as-recrystallized dye, which is much smaller than that in 1,4-dioxane. On the other hand, for YHO-2 before and after grinding of as-recrystallized dye, the absorption spectra exhibit the broadened absorption band in a visible region of 300 to 800 nm and a plateau in the range of 300 to 530 nm. However, it is difficult to provide an accurate measurement of the fluorescence spectra due to their very feeble solid-state fluorescence. Unfortunately, we could not obtain sufficient sizes of single crystals for YHO-2 and YHO-3 to make the X-ray structural analysis possible. However, for D–π–A fluorescent dyes, the red-shifts of λabsmax and λflmax, and the lowering of ΦF value by changing from solution to the solid state are quite common and explained in terms of the formation of intermolecular π–π interactions21 in the crystalline state leading to delocalization of excitons or eximers. The mismatch between the solid-state excitation spectra and absorption spectra of as-recrystallized dye YHO-3 may also be explained in terms of the formation of intermolecular π–π interactions, although the excitation spectra of YHO-2 and YHO-3 in 1,4-dioxane match closely with absorption spectra of YHO-2 and YHO-3 in 1,4-dioxane (see Fig. S1 in ESI†). Interestingly, when the ground solid of YHO-3 was melted by heating at around 150 °C, the solid after melting exhibits green metallic luster with the decrease of solid-state fluorescence property (Fig. 2b). The absorption spectrum of the solid after melting has the valley of absorption at around 560 nm, which is similar to that of the as-recrystallized dye (Fig. 3a). On the other hand, the λflmax of the solid after melting occurs at around 620 nm, which is blue-shifted by ca. 90 nm compared with that of the as-recrystallized dye (Fig. 4b). However, the solid after melting exhibited the feeble solid-state fluorescence properties (Φ ≤ 0.01). Thus, the UV-Vis diffuse reflection–absorption spectra of YHO-3 indicate that the valley of absorption at around 560 nm attributes to the appearance of green metallic luster property in both crystalline and amorphous states.
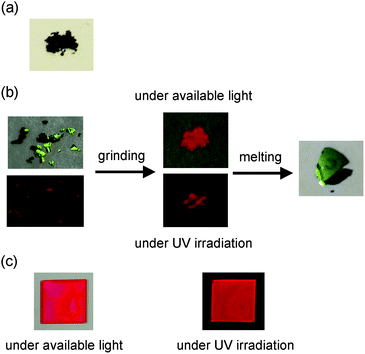 |
| | Fig. 2 Photograph of (a) YHO-2 under available light. (b) Photographs of the solids of YHO-3 under available light and under UV irradiation before and after grinding of as-recrystallized dye, and after melting the ground solids. (c) Photographs of the film of YHO-3 under available light and under UV irradiation. | |
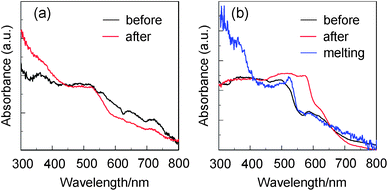 |
| | Fig. 3 (a) Solid-state UV-Vis diffuse reflection–absorption spectra of YHO-2 before and after grinding of as-recrystallized dye. (b) Solid-state UV-Vis diffuse reflection–absorption spectra of YHO-3 before and after grinding of as-recrystallized dye, and after melting the ground solids. | |
 |
| | Fig. 4 (a) Solid-state excitation and (b) fluorescence spectra of YHO-3 before (λex = 510 nm) and after (λex = 500 nm) grinding of as-recrystallized dye, and after melting (λex = 550 nm) the ground solids. | |
Moreover, in order to investigate the metallic luster property in thin film, the thin film of YHO-3 was prepared by solution casting method. The thin film of YHO-3 did not show metallic luster property but fluorescence property (Fig. 2c). The longest wavelength absorption band with absorption peak at around 530 nm is broadened compared with that in 1,4-dioxane (Fig. 5a). Interestingly, the λflmax occurs at around 625 nm, which is significantly red-shifted by ca. 90 nm compared with that in 1,4-dioxane but is blue-shifted by ca. 88 and 25 nm, respectively, compared with those of the as-recrystallized solid and the ground solid (Fig. 5b). The solid-state fluorescence quantum yield of the thin film is 0.16, which is much smaller than that in 1,4-dioxane but is larger than those of the as-recrystallized solid and the ground solid.
 |
| | Fig. 5 (a) UV-Vis diffuse reflection–absorption spectrum and (b) solid-state excitation (⋯) and fluorescence spectra (—) of the thin film of YHO-3 (λex = 510 nm). | |
Measurements of XRD and DSC
In order to elucidate the states of solids for YHO-2 and YHO-3, X-ray powder diffraction (XRD) and differential scanning calorimetry (DSC) were performed before and after grinding of the solids, and after melting the ground solids. The XRD measurements with the as-recrystallized and the ground solids of YHO-2 exhibited very weak diffraction peaks, showing that the crystal lattice was disrupted (Fig. 6a). On the other hand, the XRD measurements with the as-recrystallized dye YHO-3 exhibited diffraction peaks ascribable to well-defined microcrystalline structures (Fig. 6b). The diffraction peak intensities after grinding weakened compared with those before grinding, showing that the crystal lattice was somewhat disrupted. The solids after melting the ground solids did not show any noticeable diffraction peaks. The TG-DTA analysis indicated the dye YHO-2 shows decomposition from around 130 °C without melting. On the other hand, the DSC analysis of YHO-3 indicated that the as-recrystallized and the ground solids for YHO-3 showed only one sharp endothermic peak associated with melting (Tm) at 137 °C (Fig. 7). On the other hand, the solids after melting the ground solids underwent only an endothermic glass transition (Tg) at 85 °C due to amorphous state, and did not show melting. Therefore, the XRD profile and DSC traces of the solids after melting the ground solids are typical of amorphous solids. Consequently, this work demonstrated that the carbazole-BODIPY dye YHO-3 with ethyl heptanoate on the carbazole ring possesses green metallic luster property in both crystalline and amorphous states.
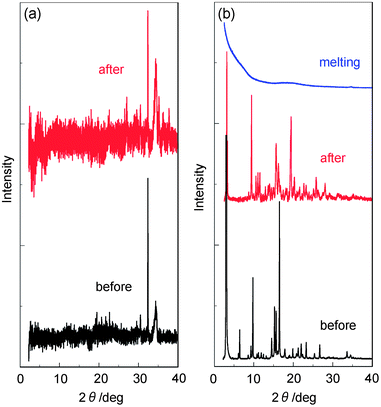 |
| | Fig. 6 (a) XRD patterns of YHO-2 before and after grinding of as-recrystallized dye. (b) XRD patterns of YHO-3 before and after grinding of as-recrystallized dye, and after melting the ground solids. | |
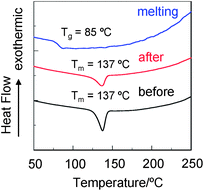 |
| | Fig. 7 DSC curves (scan rate: 10 °C min−1) of YHO-3 before and after grinding of as-recrystallized dye, and after melting the ground solids. | |
Conclusions
Carbazole-BODIPY dye YHO-3 which has a diphenylamino-carbazole moiety as an electron-donating group at the 8-position on the BODIPY core and ethyl heptanoate on the carbazole ring, was designed and synthesized and its photophysical properties in solution and in the solid state were investigated. We found that the carbazole-BODIPY dye YHO-3 possesses not only red fluorescence properties in the crystalline state but also green metallic luster properties in both crystalline and amorphous states. Moreover, this work revealed that by grinding the as-recrystallized dye YHO-3, the disappearance of the metallic luster property and the blue-shift of the fluorescence maximum are observed in the ground solid.
Experimental
IR spectra were recorded on a Perkin Elmer Spectrum One FT-IR spectrometer by ATR method. High-resolution mass spectral data were acquired on a Thermo Fisher Scientific LTQ Orbitrap XL. 1H NMR spectra were recorded on a Varian-400 FT NMR spectrometer. Absorption spectra were observed with a Hitachi U-2910 spectrophotometer and fluorescence spectra were measured with a HORIBA FluoroMax-4 spectrofluorometer. The absorption and fluorescence spectra were measured in 2.0 × 10−5 M dye/1,4-dioxane solution and 2.0 × 10−6 M dye/1,4-dioxane solution, respectively. As-recrystallized dyes were recrystallized from a mixed solvent of ethyl acetate–n-hexane. Ground samples were prepared by stressing the as-recrystallized dyes in a mortar with a pestle. Melting samples were prepared on a hot-stage with an automatic temperature control system at around 150 °C for 30 min. For the solids before and after grinding of as-recrystallized dye, and after melting the ground solids, the solid-state UV-Vis diffuse reflection–absorption spectra and solid-state fluorescence spectra were measured in quartz flat cell. Thin films of the dyes were prepared by solution casting method. Absorption spectra of the solids were observed with a Shimadzu UV-3150 spectrophotometer by using a calibrated integrating sphere system. The fluorescence quantum yields in solution and in the solid state were determined by a Hamamatsu C9920-01 equipped with CCD by using a calibrated integrating sphere system. Powder X-ray diffraction measurements were performed on a Bruker D8 diffractometer with Cu Kα radiator. Differential scanning calorimetry (DSC) of the samples was carried out using a SII EXSTAR 6000 DSC 6200.
Synthesis
10-(4-(7-(Diphenylamino)-9-(7-ethoxy-7-oxoheptyl)-9H-carbazol-2-yl)phenyl)-2,8-diethyl-5,5-difluoro-1,3,7,9-tetramethyl-5H-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-4-ium-5-uide (YHO-3).
To a mixture of 2 (ref. 19) (0.93 g, 1.57 mmol) and 2-ethyl-1,3-dimethylpyrrole (6.39 ml, 47.38 mmol) was added and trifluoroacetic acid (2.0 μl) under an argon atmosphere and stirred for 14 h at room temperature. After concentrating under reduced pressure, the resulting residue was dissolved in dichloromethane and washed with water. The organic extract was concentrated. To a solution of the residue in dichloromethane (30 ml) was added DDQ (0.39 g, 1.73 mmol) and the solution was stirred at room temperature for 12 h. Next, to the reaction mixture was added triethylamine (2.1 ml) and then BF3–OEt2 (3.06 ml, 23.55 mmol), and the solution was stirred at room temperature for 24 h. After concentrating under reduced pressure, the resulting residue was dissolved in dichloromethane and washed with water. The organic extract was concentrated. The residue was chromatographed on silica gel (hexane–ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as eluent) to give YHO-3 (0.65 g, yield 47%); IR (ATR):
1 as eluent) to give YHO-3 (0.65 g, yield 47%); IR (ATR): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1731, 1590, 1459 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 1.00 (t, J = 7.6 Hz, 6H), 1.16 (t, J = 7.2 Hz, 3H), 1.30–1.34 (m, 4H), 1.45 (s, 6H), 1.47–1.54 (m, 2H), 1.84–1.89 (m, 2H), 2.20 (t, J = 7.2 Hz, 2H), 2.34–2.40 (q, 4H), 2.52 (s, 6H), 4.01–4.06 (q, 2H), 4.42 (t, J = 6.8 Hz, 2H), 6.95 (dd, J = 2.0 and 8.4 Hz, 1H), 7.03–7.07 (m, 2H), 7.12–7.16 (m, 4H), 7.27 (d, J = 2.0 Hz, 1H), 7.29–7.34 (m, 4H), 7.49 (d, J = 8.4 Hz, 2H), 7.66 (dd, J = 1.5 and 8.0 Hz, 1H), 8.00 (d, J = 1.5 Hz, 1H), 8.07–8.10 (m, 3H), 8.19 (d, J = 8.0 Hz, 1H) ppm; 13C NMR (125 MHz, acetone-d6) δ = 12.20, 12.63, 14.58, 14.98, 17.50, 25.48, 27.45, 34.46, 43.16, 60.43 (two aliphatic carbon signals were not observed owing to overlapping resonances), 106.20, 108.06, 117.94, 119.25, 119.39, 121.07, 122.01, 123.49, 123.53, 124.68, 128.70, 129.74, 130.17, 131.56, 133.58, 135.06, 137.78, 139.21, 141.65, 142.53, 143.23, 143.33, 147.40, 149.22, 154.34, 173.56 ppm; HRMS (ESI): m/z: (M + Na+) calcd for C56H59BN4O2F2Na, 891.45813; found 891.45914.
= 1731, 1590, 1459 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 1.00 (t, J = 7.6 Hz, 6H), 1.16 (t, J = 7.2 Hz, 3H), 1.30–1.34 (m, 4H), 1.45 (s, 6H), 1.47–1.54 (m, 2H), 1.84–1.89 (m, 2H), 2.20 (t, J = 7.2 Hz, 2H), 2.34–2.40 (q, 4H), 2.52 (s, 6H), 4.01–4.06 (q, 2H), 4.42 (t, J = 6.8 Hz, 2H), 6.95 (dd, J = 2.0 and 8.4 Hz, 1H), 7.03–7.07 (m, 2H), 7.12–7.16 (m, 4H), 7.27 (d, J = 2.0 Hz, 1H), 7.29–7.34 (m, 4H), 7.49 (d, J = 8.4 Hz, 2H), 7.66 (dd, J = 1.5 and 8.0 Hz, 1H), 8.00 (d, J = 1.5 Hz, 1H), 8.07–8.10 (m, 3H), 8.19 (d, J = 8.0 Hz, 1H) ppm; 13C NMR (125 MHz, acetone-d6) δ = 12.20, 12.63, 14.58, 14.98, 17.50, 25.48, 27.45, 34.46, 43.16, 60.43 (two aliphatic carbon signals were not observed owing to overlapping resonances), 106.20, 108.06, 117.94, 119.25, 119.39, 121.07, 122.01, 123.49, 123.53, 124.68, 128.70, 129.74, 130.17, 131.56, 133.58, 135.06, 137.78, 139.21, 141.65, 142.53, 143.23, 143.33, 147.40, 149.22, 154.34, 173.56 ppm; HRMS (ESI): m/z: (M + Na+) calcd for C56H59BN4O2F2Na, 891.45813; found 891.45914.
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) (24102005 and 24550225). Y.O. also acknowledges the DIC Award in Synthetic Organic Chemistry, Japan.
Notes and references
- A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4981 CrossRef PubMed.
- G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184 CrossRef CAS PubMed.
- N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130 RSC.
- S. Hattori, K. Ohkubo, Y. Urano, H. Sunahara, T. Nagano, Y. Wada, N. V. Tkachenko, H. Lemmetyineu and S. Fukuzumi, J. Phys. Chem. B, 2005, 109, 15368 CrossRef CAS PubMed.
-
(a) S. Erten-Ela, M. D. Yilmaz, B. Icli, Y. Dede, S. Icli and E. U. Akkaya, Org. Lett., 2008, 10, 3299 CrossRef CAS PubMed;
(b) S. Kolemen, Y. Cakmak, S. Erten-Ela, Y. Altay, J. Brendel, M. Thelakkat and E. U. Akkaya, Org. Lett., 2010, 12, 3812 CrossRef CAS PubMed;
(c) S. Kolemen, O. A. Bozdemir, Y. Cakmak, G. Barin, S. Erten-Ela, M. Marszalek, J.-H. Yum, S. Zakeeruddin, M. K. Nazeeruddin, M. Grätzel and E. U. Akkaya, Chem. Sci., 2011, 2, 949 RSC.
-
(a) M. Mao, J.-B. Wang, Z.-F. Xiao, S.-Y. Dai and Q.-H. Song, Dyes Pigm., 2012, 94, 224 CrossRef CAS PubMed;
(b) J.-B. Wang, X.-Q. Fang, X. Pan, S.-Y. Dai and Q.-H. Song, Chem.–Asian J., 2012, 7, 696 CrossRef CAS PubMed.
- Y. Kubo, K. Watanabe, R. Nishiyabu, R. Hata, A. Murakami, T. Shoda and H. Ota, Org. Lett., 2011, 13, 4574 CrossRef CAS PubMed.
- R. Hu, E. Larger, A. Aguilar-Aguilar, J. Liu, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams, Y. Zhong, K. S. Wong, E. Peña-Cabrera and B. Z. Tang, J. Phys. Chem. C, 2009, 113, 15845 CAS.
- T. Ozdemir, S. Atilgan, I. Kutuk, L. T. Yildirm, A. Tulek, M. Bayindir and E. U. Akkaya, Org. Lett., 2009, 11, 2105 CrossRef CAS PubMed.
- S. Badré, V. Monnier, R. Méallet-Renault, C. Dumas-Verdes, E. Y. Schmidt, A. I. Mikhaleva, G. Laurent, G. Georges Levi, A. Ibanez, B. A. Trofimov and R. B. Pansu, J. Photochem. Photobiol., A, 2006, 183, 238 CrossRef PubMed.
- T. Vu, S. Badré, C. Dumas-Verdes, J.-J. Vachon, C. Julien, P. Audebert, E. Y. Senotrusova, E. Y. Schmidt, B. A. Trofimov, R. B. Pansu, G. Clavier and R. Méallet-Renault, J. Phys. Chem. C, 2009, 113, 11844 CAS.
- Y. Kubota, J. Uehara, K. Funabiki, M. Ebihara and M. Matsui, Tetrahedron, 2010, 51, 6195 CrossRef CAS PubMed.
- Y. Ooyama, Y. Hagiwara, T. Mizumo, Y. Harima and J. Ohshita, RSC Adv., 2013, 3, 18099 RSC.
- R. Zhao, M. Akazome, S. Matsumoto and K. Ogura, Tetrahedron, 2002, 58, 10225 CrossRef CAS.
- K. Ogura, R. Zhao, H. Yanai, K. Maeda, R. Tozawa, S. Matsumoto and M. Akazome, Bull. Chem. Soc. Jpn., 2002, 75, 2359 CrossRef CAS.
- K. Ogura, R. Zhao, M. Jiang, M. Akazome, S. Matsumoto and K. Yamaguchi, Tetrahedron Lett., 2003, 44, 3595 CrossRef CAS.
- K. Ogura, R. Zhao, T. Mizuoka, M. Akazome and S. Matsumoto, Org. Biomol. Chem., 2003, 1, 3845 CAS.
- K. Ogura, K. Ooshima, M. Akazome and S. Matsumoto, Tetrahedron, 2006, 62, 2413 CrossRef CAS PubMed.
- Y. Ooyama, Y. Hagiwara, Y. Oda, T. Mizumo, Y. Harima and J. Ohshita, New J. Chem., 2013, 37, 2336 RSC.
- Y. Ooyama, T. Sugiyama, Y. Oda, Y. Hagiwara, N. Yamaguchi, E. Miyazaki, H. Fukuoka, T. Mizumo, Y. Harima and J. Ohshita, Eur. J. Org. Chem., 2012, 4853 CrossRef CAS.
-
(a) H. Langhals, T. Potrawa, H. Nöth and G. Linti, Angew. Chem., Int. Ed. Engl., 1989, 28, 478 CrossRef;
(b) H.-C. Yeh, W.-C. Wu, Y.-S. Wen, D.-C. Dai, J.-K. Wang and C.-T. Chen, J. Org. Chem., 2004, 69, 6455 CrossRef CAS PubMed;
(c) Y. Ooyama, T. Okamoto, T. Yamaguchi, T. Suzuki, A. Hayashi and K. Yoshida, Chem.–Eur. J., 2006, 12, 7827 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra45785k |
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 M−1 cm−1 for YHO-2 and 77
600 M−1 cm−1 for YHO-2 and 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1 cm−1 for YHO-3, respectively. The absorption peak at 371 nm (ε = 39
800 M−1 cm−1 for YHO-3, respectively. The absorption peak at 371 nm (ε = 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 45
000 and 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 M−1 cm−1 for YHO-2 and YHO-3, respectively) which can be assigned to the intramolecular charge transfer (ICT) band due to phenylcarbazole-diphenylamine moiety, was also observed.13 The absorption band at around 320 nm is attributed to the local π → π* transition.20 The fluorescence maximum appears at 535 nm for YHO-2 and 536 nm for YHO-3, respectively, which occurs from the BODIPY locally excited (LE) state. The fluorescence quantum yields (Φ) of YHO-2 and YHO-3 are 0.62 and 0.70, respectively. The differences of ε and Φ values between YHO-2 and YHO-3 may be attributed to the difference in interaction between the dye and solvent, that is, the difference in affinity of dye for solvent due to carboxylic acid of YHO-2 and carboxylic acid ester for YHO-3. However, the absorption and fluorescence spectra of YHO-2 and YHO-3 resemble each other very closely, that is, this result indicates that the photophysical properties of dye themselves are similar between YHO-2 and YHO-3.
300 M−1 cm−1 for YHO-2 and YHO-3, respectively) which can be assigned to the intramolecular charge transfer (ICT) band due to phenylcarbazole-diphenylamine moiety, was also observed.13 The absorption band at around 320 nm is attributed to the local π → π* transition.20 The fluorescence maximum appears at 535 nm for YHO-2 and 536 nm for YHO-3, respectively, which occurs from the BODIPY locally excited (LE) state. The fluorescence quantum yields (Φ) of YHO-2 and YHO-3 are 0.62 and 0.70, respectively. The differences of ε and Φ values between YHO-2 and YHO-3 may be attributed to the difference in interaction between the dye and solvent, that is, the difference in affinity of dye for solvent due to carboxylic acid of YHO-2 and carboxylic acid ester for YHO-3. However, the absorption and fluorescence spectra of YHO-2 and YHO-3 resemble each other very closely, that is, this result indicates that the photophysical properties of dye themselves are similar between YHO-2 and YHO-3.




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as eluent) to give YHO-3 (0.65 g, yield 47%); IR (ATR):
1 as eluent) to give YHO-3 (0.65 g, yield 47%); IR (ATR): ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 1731, 1590, 1459 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 1.00 (t, J = 7.6 Hz, 6H), 1.16 (t, J = 7.2 Hz, 3H), 1.30–1.34 (m, 4H), 1.45 (s, 6H), 1.47–1.54 (m, 2H), 1.84–1.89 (m, 2H), 2.20 (t, J = 7.2 Hz, 2H), 2.34–2.40 (q, 4H), 2.52 (s, 6H), 4.01–4.06 (q, 2H), 4.42 (t, J = 6.8 Hz, 2H), 6.95 (dd, J = 2.0 and 8.4 Hz, 1H), 7.03–7.07 (m, 2H), 7.12–7.16 (m, 4H), 7.27 (d, J = 2.0 Hz, 1H), 7.29–7.34 (m, 4H), 7.49 (d, J = 8.4 Hz, 2H), 7.66 (dd, J = 1.5 and 8.0 Hz, 1H), 8.00 (d, J = 1.5 Hz, 1H), 8.07–8.10 (m, 3H), 8.19 (d, J = 8.0 Hz, 1H) ppm; 13C NMR (125 MHz, acetone-d6) δ = 12.20, 12.63, 14.58, 14.98, 17.50, 25.48, 27.45, 34.46, 43.16, 60.43 (two aliphatic carbon signals were not observed owing to overlapping resonances), 106.20, 108.06, 117.94, 119.25, 119.39, 121.07, 122.01, 123.49, 123.53, 124.68, 128.70, 129.74, 130.17, 131.56, 133.58, 135.06, 137.78, 139.21, 141.65, 142.53, 143.23, 143.33, 147.40, 149.22, 154.34, 173.56 ppm; HRMS (ESI): m/z: (M + Na+) calcd for C56H59BN4O2F2Na, 891.45813; found 891.45914.
= 1731, 1590, 1459 cm−1; 1H NMR (400 MHz, acetone-d6) δ = 1.00 (t, J = 7.6 Hz, 6H), 1.16 (t, J = 7.2 Hz, 3H), 1.30–1.34 (m, 4H), 1.45 (s, 6H), 1.47–1.54 (m, 2H), 1.84–1.89 (m, 2H), 2.20 (t, J = 7.2 Hz, 2H), 2.34–2.40 (q, 4H), 2.52 (s, 6H), 4.01–4.06 (q, 2H), 4.42 (t, J = 6.8 Hz, 2H), 6.95 (dd, J = 2.0 and 8.4 Hz, 1H), 7.03–7.07 (m, 2H), 7.12–7.16 (m, 4H), 7.27 (d, J = 2.0 Hz, 1H), 7.29–7.34 (m, 4H), 7.49 (d, J = 8.4 Hz, 2H), 7.66 (dd, J = 1.5 and 8.0 Hz, 1H), 8.00 (d, J = 1.5 Hz, 1H), 8.07–8.10 (m, 3H), 8.19 (d, J = 8.0 Hz, 1H) ppm; 13C NMR (125 MHz, acetone-d6) δ = 12.20, 12.63, 14.58, 14.98, 17.50, 25.48, 27.45, 34.46, 43.16, 60.43 (two aliphatic carbon signals were not observed owing to overlapping resonances), 106.20, 108.06, 117.94, 119.25, 119.39, 121.07, 122.01, 123.49, 123.53, 124.68, 128.70, 129.74, 130.17, 131.56, 133.58, 135.06, 137.78, 139.21, 141.65, 142.53, 143.23, 143.33, 147.40, 149.22, 154.34, 173.56 ppm; HRMS (ESI): m/z: (M + Na+) calcd for C56H59BN4O2F2Na, 891.45813; found 891.45914.




