(S)-BINOL-based boronic ester fluorescence sensors for enantioselective recognition of α-phenylethylamine and phenylglycinol†
Abstract
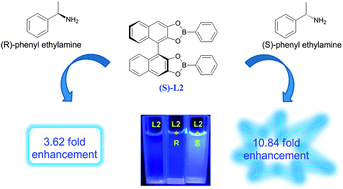
Four chiral fluorescence sensors (S)-L1–4 incorporating boronic ester and (S)-1,1′-bi-2-naphthol (BINOL) moieties were synthesized and developed for the enantioselective recognition of α-phenylethylamine and phenylglycinol enantiomers. The sensor (S)-L1 shows an obvious fluorescence quenching “turn-off” response towards enantiomers of both α-phenylethylamine and phenylglycinol. Interestingly, the sensor (S)-L2 can exhibit remarkable fluorescent enhancement “turn-on” response behavior towards (S)-α-phenylethylamine, but shows no response towards phenylglycinol enantiomers. The Stern–Volmer constant (Ksv) values of (S)-L1 are 0.63 × 103 L mol−1 and 4.48 × 103 L mol−1 for (L)- and (D)-phenylglycinol, respectively, and the value of the enantiomeric fluorescence difference ratio (ef) of (S)-L2 is 4.6 for α-phenylethylamine, demonstrating that (S)-L2 could be used as a fluorescence sensor for simple and direct visual discrimination of organic molecule enantiomers. On the contrary, no fluorescence enantioselective recognition response could be observed when using the (S)-BINOL-based boronic ester sensors (S)-L3 and (S)-L4 incorporating bigger naphthyl or 8-methoxyquinolinyl substituent groups.

 Please wait while we load your content...
Please wait while we load your content...