Graphene sheets anchored with high density TiO2 nanocrystals and their application in quantum dot-sensitized solar cells†
Abstract
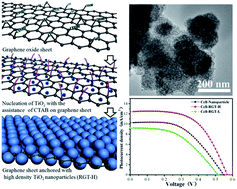
High density TiO2 nanocrystals were successfully decorated on graphene sheets via a CTAB assisted solvothermal method. Due to the structural advantages, an improved power conversion efficiency of 4.02% was achieved in a quantum dot-sensitized solar cell, demonstrating ∼40% enhancement compared with the nanoparticle based cell.

 Please wait while we load your content...
Please wait while we load your content...