Multifunctional poly(ester urethane) laminates with encoded information†
Melanie
Ecker
and
Thorsten
Pretsch
*
BAM Federal Institute for Materials Research and Testing, Division 6.5, Polymers in Life Science and Nanotechnology, Unter den Eichen 87, 12205 Berlin, Germany. E-mail: thorsten.pretsch@bam.de; Fax: +49 30 8104-1617; Tel: +49 30 8104-3804
First published on 20th November 2013
Abstract
Solvent-cast films from shape memory poly(ester urethane) (PEU) containing different weight contents of microencapsulated thermochromic pigments (T-PIGs) were prepared by drying in air. Spectrophotometric investigations unveiled that gradual loading with T-PIG black resulted in continuous darkening of the films up to filler contents of 20 wt%, accompanied by a steady enhancement of thermochromic properties. Taking this composition as standard, PEU films equipped with T-PIG black, blue and red were deposited atop PEU plaques to obtain laminate structures. Herein, the cover layer thickness (100 ± 5 μm) and the good dispersion of T-PIGs inside the polymer matrix were verified by scanning electron microscopy. Machine-readable information carriers were prepared by laser engraving quick response (QR) codes into the cover layer of the laminates and subsequently cutting cuboidal samples therefrom. Finally, thermo-mechanical programming of the QR code carriers was applied to randomly distort the code patterns, thus rendering them unreadable. Upon heating, surface decolorization and shape recovering occurred; during the ensuing cooling, the surface color and contrast reappeared whereupon the QR codes could be read out. Spectrophotometric, calorimetric and thermo-mechanical investigations gave evidence that the color switching temperature of the T-PIGs roughly coincided with the melting temperature of the ester-based switching segment and thus with the activation temperature of the shape memory effect. Apart from that unique functionality, manifold design concepts may render information carriers difficult-to-copy. Therefore, we anticipate tremendous potential as anti-counterfeiting technology.
Introduction
Shape memory polymers (SMPs) belong to the class of smart materials. Once thermo-mechanically programmed (functionalized), a temporary shape is maintained until the shape memory effect (SME) is thermally triggered, which usually results in the almost complete recovering of the permanent shape. When equipped with appropriate fillers, exposure to an alternating magnetic field or the application of an electrical current even enable an indirect triggering of the thermally induced SME.1–4 Further stimuli include light (in case of photo-responsive SMPs)5 and the diffusion of low molecular weight plasticizers into SMPs.6,7 Shape memory polymers and their composites have become increasingly important in the past few years;8–14 various examples of use indicate a blurring of the borderline between research and applications.15–24Physically cross-linked block copolymers like poly(ester urethane) (PEU) may have distinct shape memory properties. In such phase segregated systems, hard segments act as net points whereas soft segments serve as mobile phase.25,26 Depending on the underlying thermal transition and programming conditions like deformation and shape fixity temperature, PEU can change shape as a result of soft segment devitrification and melting.27 One further advantage of PEU consists in a chemically tunable soft segmental melting transition, which can be set e.g. at around 40 °C and thus slightly above room temperature.28
Recently, Desmopan DP 2795A SMP, a PEU from Bayer MaterialScience AG, proved to be particularly suitable as base material for switchable information carriers.29 For instance, two-dimensional (2D) codes like quick response (QR), Data Matrix and Aztec can be engraved into surface-dyed PEU plates. When subsequently programmed, these codes become unreadable and keep that state until the SME is triggered by heating, whereupon the encoded information can be easily read out with a scanning and decoding device. So far, reliable shape change behavior grants access to hitherto hidden or distorted information, which qualifies information carriers as useful technology for anti-counterfeit product verification and identification.30
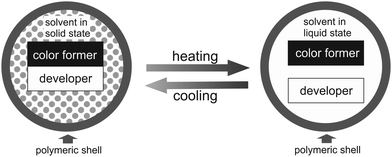
In search of one further, thermo-responsive security feature, fitting well to our information carrier concept, we became aware of thermochromic dyes.31,32 Microencapsulated thermochromic pigments (T-PIGs) may turn colorless when heated above the color switching temperature (CST) and regain color upon cooling below that temperature.33 When utilizing melamine resin as polymeric shell material, the coloring components may include an electron-donating, chromogenic organic compound (color former, in our case leuco dye) and an electron-accepting agent (developer, commonly a weak organic acid like hexa- or octadecanol); in addition, a solvent (long chain alkyl alcohol, ester or acid) is used as matrix component (Fig. 1).34,35
Since the thermochromic mixture usually contains about 75–95 mol% solvent (only low concentrations of color former, usually about 1 mol%, are needed due to the high extinction coefficient of the dye), the melting and crystallization points of the solvent largely control that of the mixture.36 Below the solvent melting temperature, the coloring components of thermochromic leuco dye-developer-solvent systems electronically interact, forming an extended conjugated π-electron system.37 Upon a temperature increase above the solvent melting transition, the color-forming components get separated from each other, whereupon electronic interaction is inhibited, thus rendering the microcapsules achromatic.38,39 Reliable color switch functionality is ensured as long as the protective polymeric microcapsules remain undamaged.
In this contribution, we aimed at bringing together both functionalities in one and the same system – shape memory behavior and thermochromism. Therefore, we developed pastes, containing PEU as SMP, T-PIGs and a solvent for PEU. In contrast to recent research efforts by Jinlian Hu and her colleagues, who elegantly took advantage of the adhesiveness of (achromatic) urethane-based solutions by connecting two layers of urethane-based SMP, one programmed the other one in permanent shape,40,41 we will demonstrate that paste preparation is a useful technique to obtain thermochromic films, which may even be tightly connected to PEU plaques, forming structurally stable laminates. These polymer bilayer structures were taken as basis to produce QR code carriers therefrom, characterized by an exceptional combination of shape memory and color switch functionality.
Experimental section
Fabrication methods
Desmopan DP 2795A SMP, a poly(ester urethane) (PEU) from Bayer MaterialScience AG, was used as base material for the fabrication of multifunctional information carriers. The hard segments were composed of 4,4′-methylenediphenyl diisocyanate and 1,4-butanediol as chain extender. The soft segments were based on poly(1,4-butylene adipate) (PBA). The raw material was supplied both as pellets and cuboidal, injection molded plaques with dimensions of 126 mm × 52 mm × 2 mm.At first, different PEU pastes, containing microencapsulated thermochromic pigment powder (T-PIG, Sintal Chemie GmbH) of black, blue or red room temperature color, were prepared. The mass densities of the T-PIGs were 0.26 g cm−3. For paste preparation, 5 g of PEU were dissolved in 15 ml of N-methyl-2-pyrrolidone (NMP), before 1.25 g of T-PIG was added to the viscous solution. The obtained liquid paste was well stirred for 5 min to homogenously disperse the T-PIG. Following a solvent casting approach, the paste was either layered atop glass plates to prepare PEU films filled with T-PIG black, blue and red or layered atop PEU plaques to fabricate laminate structures. Here, an applicator was used to homogeneously distribute the liquid paste on the upper PEU surface, before drying on air at 23 °C was carried out for about 18 h. A CO2 laser was used to fabricate information carriers therefrom. The main steps included engraving a QR code into the pigment-containing PEU layer and cutting cuboid samples with a dimensioning of 25 mm × 25 mm × 2 mm. In order to ensure sufficiently high surface contrast in the obtained QR code carriers at room temperature, the laser ablation depth was set to exceed the deposition height of the cover layer. In all cases, the QR codes carried the encoded information “http://www.bam.de/en/kompetenzen/fachabteilungen/abteilung_6/fg65/fb65_formgedaechtnispolymere.htm.”
Characterization methods
A scanning electron microscope (SEM) was used to study both the shape and size distribution of the T-PIGs and the surface morphology of the laminates. For this purpose, a Zeiss Gemini Supra 40 device, operating at an extra high tension of 10 kV, was employed. By means of a sputtering system, powdery T-PIGs were coated with a few nanometers thin carbon layer, whereas the laminates were coated with a thin layer of gold. Cross sections of the laminates were prepared at −20 °C using a Leica CM1950 device. The size distribution of the T-PIGs was determined from scanning electron micrographs. By standard, 500 particles were analyzed with the program ImageJ.Spectrophotometric investigations were conducted with the modular spectrometer system MultiSpec UV-VIS-NIR from tec5. The experimental setup is provided by Fig. S1, ESI†. The spectrometer operated in the wavelength scan range between 730 and 360 nm. The reflection properties of PEU films loaded with T-PIG black, blue and red were determined at different temperatures, 50% air humidity and a measuring geometry of 45°/0°. Inside the climate chamber (VCL 4006, Vötsch Industrietechnik GmbH), the loaded films were heated from 23 to 55 °C with rates of 0.4 °C min−1, before they were cooled to 23 °C with rates of 0.3 °C min−1. The sample temperature was followed with a Pt100 temperature sensing device, which was connected to the backside of each sample. To ensure efficient heat transfer, a heat-conductive paste (Keratherm KP12) was used at the contact areas. The D65 standard illuminant and 2° standard observer were selected to determine the colorimetric properties of the samples from the reflectance data by using the mathematically defined CIE 1976 L*a*b* (CIELAB) color space.42–44 From the CIELAB values, the temperature-dependent color difference ΔE as defined in eqn (1) was calculated for PEU films loaded with T-PIG black, blue and red.
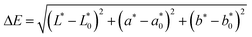 | (1) |
The ΔE values were determined with reference to the CIELAB values at the respective starting temperatures, which were 23 °C on heating and 55 °C on cooling. The color switching temperature (CST) was defined as that temperature, at which the strongest change in ΔE occurred, coinciding with the peak maximum in the ΔE/dT derivative.
The mechanical properties of solvent-cast, non-loaded PEU films and solvent-cast PEU films loaded with 20 wt% of T-PIG black were investigated with an MTS Insight 10 electromechanical testing system. The studied films had a dimensioning of 50 mm × 5 mm × 0.1 mm. The tensile tests were carried out 72 h after film preparation. The films were clamped, using a clamping distance of 10 mm, and stretched at 23 °C with a deformation rate of 30 mm min−1 until failure occurred. The tensile data was averaged from three independent measurements.
Differential scanning calorimetry (DSC) was used to characterize both as-received powdery T-PIGs and solvent-cast films of PEU and PEU loaded with T-PIGs, respectively. The measurements were carried out with a DSC 7020 from Seiko. Initially, a sample, weighing approximately 5 mg, was sealed in a DSC aluminum pan, before it was placed inside the calorimeter. The sample was cooled down to −80 °C, before it was heated to 90 °C. Subsequently, the sample was cooled to −80 °C and heated for a second time to 90 °C. For all measurements, temperature holding times of 5 min at −80 and 90 °C were selected and uniform heating and cooling rates of 10 °C min−1 chosen.
To determine the surface contrast in multifunctional QR code carriers, color images were taken and converted into grayscale histograms by using the image analysis program ImageJ.45 From these results, the Michelson contrast CM between surface-colored regions (containing T-PIG) and laser-ablated regions (PEU substrate) was determined at 23 and 60 °C. For this purpose, CM was defined as relation between the difference and the sum of the highest and lowest luminance Lmax and Lmin, differing in grayscale value (GSV).46
The programming of multifunctional QR code carriers was carried out with an MTS Insight 10 electromechanical testing system, which was equipped with a Thermcraft thermochamber. Heating of the information carriers was accomplished by two electrical heating elements in the back of the thermochamber, whereas cooling was achieved by injecting liquid nitrogen from a Dewar vessel into the thermochamber. For simplicity a recently introduced programming method (compressive deformation-determinated functionalization29) was used. In the experimental setup, the multifunctional QR code carrier was positioned in the center of an unmovable pressure plate with its laser engraved site pointing toward a second pressure plate, which was connected over steel rods to the above moveable crosshead. After applying an initial force of −10 N, the information carrier was heated from 23 to 80 °C with a rate of 4.3 °C min−1 and thus above the DSC melting peak offset of the PBA phase (which was close to 50 °C).47 After 5 min at 80 °C, the QR code carrier was loaded with a crosshead displacement rate of 0.5 mm min−1 up to a maximum force Fmax of 3 kN. In order to fix the temporary shape, characterized by a strongly distorted QR code pattern, Fmax was maintained during cooling with a rate of 5.3 °C min−1 to −20 °C and thus below the DSC crystallization offset temperature of the PBA phase transition (around −10 °C).47 After 5 min at −20 °C, the QR code carrier was unloaded with a rate of 500 N min−1, before it was heated to 23 °C. In order to trigger the shape memory effect and recover the original shape, the PEU was reheated to 80 °C and kept there for 5 min. Finally, the QR code carrier was cooled to 23 °C.
The machine-readability of freshly prepared, programmed and recovered multifunctional QR code carriers was investigated with a Samsung Galaxy S I9000 smartphone, equipped with the software “Barcode Scanner” version 4.2 from ZXing. Most often, the QR codes were scanned at 23 and 60 °C, thus at temperatures below and above the CST of the T-PIG.
Results and discussion
First of all, we investigated the shape and size distribution of as-received powdery T-PIGs, which we later used as filler materials for PEU. A detailed view on the surface morphology of the polymeric microcapsules is provided by Fig. 2.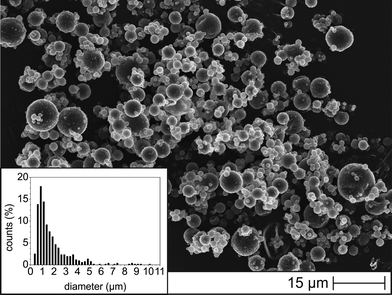 | ||
| Fig. 2 SEM image of as-received powdery T-PIG black; the inset gives the size distribution of the microcapsules. | ||
The SEM image clearly reveals microcapsules of spherical geometries, characterized by a polydisperse size distribution, spreading from 0.3 to 10 μm. The mean particle size was about 1.8 μm. A similar size distribution could be detected for as-received T-PIG blue; in case of T-PIG red, the average particle size was lowest (Fig. S2, ESI†).
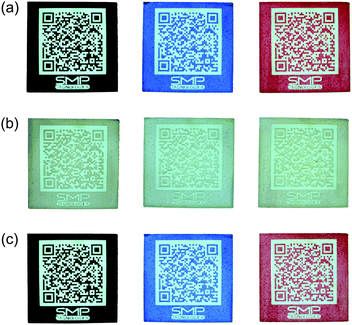
In a next step, PEU pastes with various contents of T-PIG black were prepared and layered atop glass plates. Evaporation of the solvent NMP resulted in the formation of homogenously colored films, whose room temperature color intensity grew with rising filler contents. All films had in common, that uniform discoloration was detected upon heating to 60 °C (Fig. 3).
The reversible nature of color switching between the black and colorless dye forms was proven in ten successive thermal cycles.
In a subsequent step, the thermochromic films were spectrophotometrically investigated at 23 °C. As evidenced by the evolution of CIELAB lightness L* (Fig. S3, ESI†), gradual loading with T-PIG black resulted in a continuous darkening of the films up to filler contents of 20 wt%. Here, the difference in lightness was strongest, tantamount to highest contrast between the unloaded and loaded PEU forms. This finding suggests a promising composition to realize distinct thermochromic effects. Therefore, we decided to prepare PEU films, containing 20 wt% of T-PIG black, blue and red, and to investigate them in continuative studies. Before concentrating on the functional performance of the employed fillers, we carried out tensile tests on solvent-cast PEU films. As expected, adding 20 wt% of T-PIG black to the PEU matrix resulted in a substantial enhancement in mechanical properties. Compared to non-loaded PEU film, increases in Young's modulus (from 2.7 ± 1.1 to 44.7 ± 9.0 MPa), ultimate tensile strength (from 4.6 ± 1.2 to 16.1 ± 2.2 MPa) and toughness (from 32.3 ± 8.4 to 138.2 ± 17.4 MJ m−3) could be detected. Thus, the above selected composition proved also useful from a mechanical point of view. Next, we investigated the temperature-dependent colorimetric properties of our loaded PEU films (Fig. S4, ESI† and Table 1).
| T-PIG | CST (heating) | CST (cooling) | ||||
|---|---|---|---|---|---|---|
| Onset (°C) | Max. (°C) | Offset (°C) | Onset (°C) | Max. (°C) | Offset (°C) | |
| Black | 31 | 38 | 43 | 40 | 32 | 30 |
| Blue | 26 | 40 | 45 | 40 | 34 | 30 |
| Red | 29 | 42 | 47 | 40 | 33 | 24 |
As effectively controlled by the solvent melting and crystallization behavior inside the polymeric microcapsules, the films decolorized upon heating at temperatures between 43 and 47 °C and regained their original color on cooling at temperatures between 30 and 24 °C.
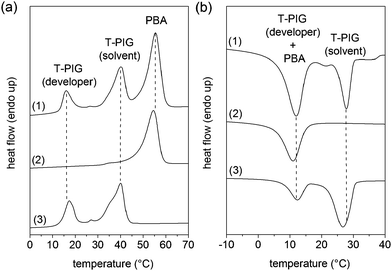
Another important aspect in the investigation of functional polymer films is their calorimetric properties, which have been determined by DSC (Fig. 4).
In the DSC thermograms, the peak positions of the endo- and exothermic signals of PEU film exemplarily loaded with 20 wt% of T-PIG black only slightly deviated from those taken from solvent-cast, non-loaded PEU film and as-received powdery T-PIG black. Similarly characteristic melting and crystallization patterns were detected in the respective thermograms of PEU loaded with T-PIG blue and red (Table S1†). In detail, the DSC melting and crystallization peak temperatures of the PBA phase were detected at around 55 and 11 °C. Compared to the phase transition temperatures of injection molded PEU plaques,47 the values were slightly raised, suggesting that processing affected the thermal stability of the PBA phase. Independent of the filler content, loading PEU films with T-PIG black resulted in slightly decreased PBA melting enthalpies and therefore reduced PBA crystallinities (Table S1†). Obviously, the interaction of the melamine microcapsules with PBA chains restricted segmental motions and therefore promoted noncrystalline PBA conformations. It is reasonable to expect that physical adsorption of PBA chains to the melamine shell of the microcapsules can slow down the polymer dynamics, which might also result in an increase in the PBA glass transition temperature.48 Nevertheless, there was no clear hint therefore, since Tg (PBA) was approximately −44 °C for both loaded and unloaded PEU. In the DSC thermograms, the remaining signals were attributed to solvent phase changes inside the thermochromic microcapsules. Herein, the solvent melted at 40 °C and crystallized at 28 °C as indispensable for the reliable color switch functionality of the T-PIGs and in pretty good agreement with our colorimetric results (Table 1). The mean size of the microcapsules is known to affect the crystallization behavior of solvents like n-octane in DSC studies.49 However, in our case the absolute difference in average particle size was 0.6 μm (Fig. S2, ESI†) and appeared to be too small to influence the phase transition behavior of the solvent in T-PIG black, blue and red (Table S1†). Beyond that, heating- and cooling-related signals at 19 and 13 °C were attributed to the melting and crystallization of the weak organic acid (developer).
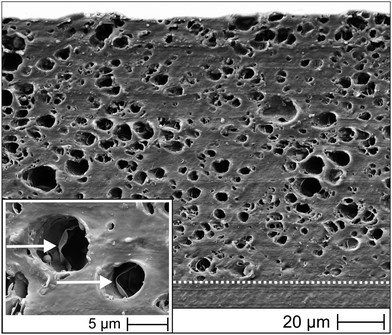
To fabricate laminate structures, PEU plaques were coated with thermochromic PEU pastes containing 20 wt% of micron-size fillers. A representative SEM image of the cover layer and its transition zone with the PEU substrate is provided by Fig. 5.
The cover layer thickness was about (100 ± 5) μm. On closer consideration, almost spherical and randomly distributed cavities are visible, which we assigned to those volumes filled with T-PIGs (SEM images of solvent-cast PEU did not exhibit cavities at all). In other words, the applied solvent casting technique and the selected filler/matrix ratio ensured a fairly good dispersion of functional dye inside the PEU matrix. The diameters of the cavities spread from 1 to 8 μm and were thus in the same range as the size distribution of the microcapsules (Fig. 2). At a higher resolution, even the walls of the microcapsules, which were cut open in course of cryomicrotome sectioning, could be visualized (marked by arrows in Fig. 5). It is remarkable that no sharp interface between the PEU substrate and the cover layer could be detected, implying that a tightly connected bilayer structure was formed.
Finally, we prepared multifunctional information carriers by laser engraving QR codes into the thermochromic PEU cover layers of our laminates (Fig. 6). Therefore, the laser ablation depth was set to exceed the height of the cover layer by (10 ± 5) μm.
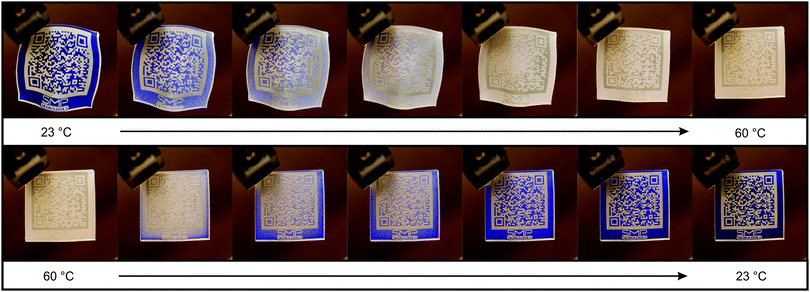
 | ||
| Fig. 6 Multifunctional QR code carriers at 23 °C (a), 60 °C (b) and 23 °C (c). The QR codes were consistently machine-readable below CST (a and c) and unreadable above CST (b). | ||
Since the PEU base material was semi-crystalline at room temperature and therefore whitish, an adequate surface contrast was assured between opaque regions, which “laid open” after laser ablation, and non-laser ablated (colorful) regions. As a consequence, the QR codes were consistently machine-readable at 23 °C (Fig. 6a and c). In equal measure, color fading and an associated contrast decline gave non-decodable states above CST (Fig. 6b). In this sense, the cover layer exhibited reliable thermochromic properties.
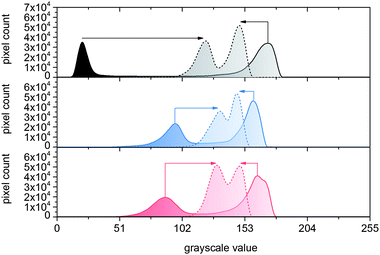
As illustrated in the grayscale histograms, pronounced contrast changes could be verified for multifunctional QR code carriers when heated from 23 to 60 °C (Fig. 7).
Two characteristic signal shifts took place in the grayscale histograms when heating information carriers with black and white QR code patterns above CST. In terms of the black areas, decolorization occurred as evidenced by a shift in GSV from 20 to 121. In parallel, a slight darkening of whitish regions, illustrated by a shift from 172 to 148, could be detected. While the former is attributable to the separation of color-forming components, culminating in decolorization of T-PIG black, latter is due to PBA melting. Similarly characteristic, but less pronounced shifts in GSV were found for QR code carriers, whose surfaces were loaded with T-PIG blue and red. Independent of the employed fillers, the Michelson contrast in black, blue and red QR code carriers was much higher below CST (CM = 0.25–0.80) compared to above CST (CM = 0.04–0.09). In agreement with recent investigations on artificially aged information carriers surface-loaded with non-functional dye,45 a drop below CM = 0.1 led to the malfunction of QR code readability.
For convenience, programming of those multifunctional QR code carriers shown in Fig. 6 was carried out via compressive deformation-determinated functionalization.29 Hence, the QR codes became non-decipherable, since the deskewing and decoding algorithms of the scanning and decoding device could not compensate the random distortion patterns (not shown in Fig. 6). For all investigated QR code carriers, the characteristic surface coloring did not change compared to their pre-programmed states, which implies that the microcapsules did not burst in course of mechanical loading and were therefore sufficiently mechanical stable.50 The processes of shape recovering and surface decolorization are exemplified in Fig. 8 for a blue QR code carrier, same as the ensuing cooling-initiated return of surface contrast. As visible to the naked eye, decolorization of those surface regions containing T-PIG blue set in slightly before shape recovering. This finding is in line with our DSC results where solvent melting inside the thermochromic microcapsules was detected below the PBA melting transition, which herein corresponds to the shape recovery temperature. At 60 °C, the original shape was completely recovered, suggesting that the influence of thermochromic additives, counteracting the entropy elastic behavior of polymer chains, was negligible. However, at that temperature the QR code was still machine unreadable due to a lack of surface contrast. Once cooled below CST, the contrast returned and the encoded information could be released. Finally, it is noteworthy that multifunctional QR code carries were characterized by fairly good structural stabilities; the cover layers containing the microcapsules even did not peel off, when keeping the QR code carriers for more than one week at 60 °C.
Conclusions
In summary, the deposition of thermochromic PEU films atop PEU plaques is a convenient way to obtain polymer laminate structures, which can be used to fabricate multifunctional QR code carriers therefrom. Advantageously, the concepts of thermochromism and shape memory behavior complemented one another due to an excellent switching behavior. While significant advances have been made in developing information carriers with combinatorial thermo-responsiveness, there are still numerous challenges ahead to bring this promising technology from the bench into applications. One of them is integrating information carriers into existing systems. For applications mandating programmed information carriers that are precisely the same size and shape, this challenge may actually be a limitation. However with the introduction of the first generation of multifunctional information carriers, a significant number of fundamental and practical challenges could be solved, which makes us optimistic that in practice, the full potential of this remarkable technology can be realized one day.Acknowledgements
The authors gratefully acknowledge financial support from the German Federal Ministry of Education and Research (BMBF, project funding reference number 03V0043). Daffne Pilger Vaca is acknowledged for programming and recovering the information carriers, Sigrid Benemann for conducting SEM and Sebastian Chruscicki for colorimetric measurements. The authors thank Bayer MaterialScience AG for kindly providing the PEU.Notes and references
- Y. Liu, H. Lv, X. Lan, J. S. Leng and S. Du, Compos. Sci. Technol., 2009, 69, 2064–2068 CrossRef CAS PubMed.
- S. A. Madbouly and A. Lendlein, Adv. Polym. Sci., 2010, 226, 41–95 CrossRef CAS.
- Q. Meng and J. Hu, Composites, Part A, 2009, 40, 1661–1672 CrossRef PubMed.
- J. Leng, X. Lan, Y. Liu and S. Du, Prog. Mater. Sci., 2011, 56, 1077–1135 CrossRef CAS PubMed.
- A. Lendlein, H. Jiang, O. Jünger and R. Langer, Nature, 2005, 434, 879–882 CrossRef CAS PubMed.
- W. M. Huang, B. Yang, Y. Zhao and Z. Ding, J. Mater. Chem., 2010, 20, 3367–3381 RSC.
- X. Wu, W. M. Huang, Y. Zhao, Z. Ding, C. Tang and J. Zhang, Polymers, 2013, 5, 1169–1202 CrossRef.
- P. T. Mather, X. Luo and I. A. Rousseau, Annu. Rev. Mater. Res., 2009, 39, 445–471 CrossRef CAS.
- M. Behl, J. Zotzmann and A. Lendlein, Adv. Polym. Sci., 2010, 1–40 Search PubMed.
- T. Pretsch, Polymers, 2010, 2, 120–158 CrossRef CAS.
- L. Sun, W. M. Huang, Z. Ding, Y. Zhao, C. C. Wang, H. Purnawali and C. Tang, Mater. Des., 2012, 33, 577–640 CrossRef CAS PubMed.
- K. K. Julich-Gruner, C. Löwenberg, A. T. Neffe, M. Behl and A. Lendlein, Macromol. Chem. Phys., 2013, 214, 527–536 CrossRef CAS.
- H. Meng and G. Li, Polymer, 2013, 54, 2199–2221 CrossRef CAS PubMed.
- H. Meng, H. Mohamadian, M. Stubblefield, D. Jerro, S. Ibekwe, S.-S. Pang and G. Li, Smart Mater. Struct., 2013, 22, 093001 CrossRef.
- A. Lendlein, M. Behl, B. Hiebl and C. Wischke, Expert Rev. Med. Devices, 2010, 7, 357–379 CrossRef CAS PubMed.
- J. Hu and S. Chen, J. Mater. Chem., 2010, 20, 3346–3355 RSC.
- W. Small, P. Singhal, T. S. Wilson and D. J. Maitland, J. Mater. Chem., 2010, 20, 3356–3366 RSC.
- M. Ebara, K. Uto, N. Idota, J. M. Hoffmann and T. Aoyagi, Adv. Mater., 2012, 24, 273–278 CrossRef CAS PubMed.
- J. Hu, H. Meng, L. Guoqiang and S. I. Ibekwe, Smart Mater. Struct., 2012, 21, 053001 CrossRef.
- Y. Liu, J. K. Boyles, J. Genzer and M. D. Dickey, Soft Matter, 2012, 8, 1764–1769 RSC.
- M. C. Serrano and G. A. Ameer, Macromol. Biosci., 2012, 12, 1156–1171 CrossRef CAS PubMed.
- S. Wang and J. C. Brigham, Smart Mater. Struct., 2012, 21, 105016 CrossRef.
- M. Anthamatten, S. Roddecha and J. Li, Macromolecules, 2013, 46, 4230–4234 CrossRef CAS.
- A. Gugliuzza and E. Drioli, J. Membr. Sci., 2013, 446, 350–375 CrossRef CAS PubMed.
- B. K. Kim, S. Y. Lee and M. Xu, Polymer, 1996, 37, 5781–5793 CrossRef CAS.
- F. L. Ji, J. L. Hu, T. C. Li and Y. W. Wong, Polymer, 2007, 48, 5133–5145 CrossRef CAS PubMed.
- T. Pretsch, Smart Mater. Struct., 2010, 19, 015006 CrossRef.
- M. Bothe, F. Emmerling and T. Pretsch, Macromol. Chem. Phys., 2013 DOI:10.1002/macp.201300464.
- T. Pretsch, M. Ecker, M. Schildhauer and M. Maskos, J. Mater. Chem., 2012, 22, 7757–7766 RSC.
- J. Hättig, W. Bräuer and T. Pretsch, Kunststoffe international, 2013, 1, 39–41 Search PubMed.
- A. Seeboth and D. Lötzsch, Thermochromic Phenomena in Polymers, Smithers Rapra, Shawbury, Shrewsbury, Shropshire, 2008 Search PubMed.
- A. Seeboth, R. Ruhmann and O. Mühling, Materials, 2010, 3, 5143–5168 CrossRef CAS.
- R. Kulčar, M. Friškovec, N. Hauptman, A. Vesel and M. Klanjšek Gunde, Dyes Pigm., 2010, 86, 271–277 CrossRef PubMed.
- S. Lakio, J. Heinämäki and J. Yliruusi, AAPS PharmSciTech, 2010, 11, 46–53 CrossRef CAS PubMed.
- R. Kulčar, M. Friškovec, M. Klanjšek Gunde and N. Knešaurek, Color. Technol., 2011, 127, 411–417 Search PubMed.
- D. C. MacLaren and M. A. White, J. Mater. Chem., 2003, 13, 1695–1700 RSC.
- C. F. Zhu and A. B. Wu, Thermochim. Acta, 2005, 425, 7–12 CrossRef CAS PubMed.
- A. Seeboth, D. Lötzsch, E. Potechius and R. Vetter, Chin. J. Polym. Sci., 2006, 24, 363–368 CrossRef CAS.
- A. Seeboth, A. Klukowska, R. Ruhmann and D. Lötzsch, Chin. J. Polym. Sci., 2007, 25, 123–135 CrossRef CAS.
- S. Chen, J. Hu, H. Zhuo and Y. Zhu, Mater. Lett., 2008, 62, 4088–4090 CrossRef CAS PubMed.
- S. Chen, J. Hu and H. Zhuo, Compos. Sci. Technol., 2010, 70, 1437–1443 CrossRef CAS PubMed.
- DIN, Measuring conditions for object colors, German Institute for Standardization, Berlin, Germany, 1983, vol. DIN 5033-7.
- CIE, in 3rd Edition, Commission Internationale de l'Eclairage, Vienna, Austria, 2004, vol. CIE 15.3:2004.
- CIE, Commission Internationale de l'Eclairage, Vienna, Austria, vol. CIE S 017/E:2011.
- M. Ecker and T. Pretsch, Smart Mater. Struct., 2013, 22, 094005 CrossRef.
- A. A. Michelson, Studies in Optics, The University of Chicago Press, Chicago, Illinois, 1927 Search PubMed.
- T. Pretsch, I. Jakob and W. Müller, Polym. Degrad. Stab., 2009, 94, 61–73 CrossRef CAS PubMed.
- C. G. Robertson, C. J. Lin, M. Rackaitis and C. M. Roland, Macromolecules, 2008, 41, 2727–2731 CrossRef CAS.
- X.-X. Zhang, Y.-F. Fan, X.-M. Tao and K.-L. Yick, J. Colloid Interface Sci., 2005, 281, 299–306 CrossRef CAS PubMed.
- G. Sun and Z. Zhang, J. Microencapsulation, 2001, 18, 593–602 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental setup for spectral analysis, size distributions for as-received T-PIG blue and red, CIELAB lightness for PEU films differently loaded with T-PIG black, temperature-depending colorimetric properties of PEU films loaded with T-PIG black, blue and red and calorimetric data. See DOI: 10.1039/c3ra45651j |
| This journal is © The Royal Society of Chemistry 2014 |