Moving towards individualized medicine with microfluidics technology
Abstract
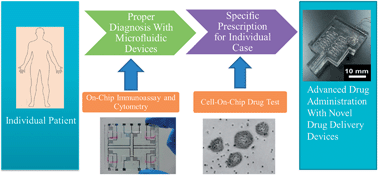
The development of microfluidics technology has enabled the biomedical research community to create novel strategies for applications ranging from diagnostics to therapy of various human diseases. Recent advances in microfluidic technology will aid in providing new sets of solutions to overcome the shortcomings of conventional detection and treatment methods available in clinics and hospitals. Microfluidic technology is equipped with the ability to precisely control and manipulate fluids and allow medical researchers to engineer a translational medicine platform for rapid biological sample analysis and controlled drug delivery therapy. In addition, the dimensions of microfluidic device can be miniaturized to a desirable size thereby offering the convenience of embedded implant treatment. These unique features of microfluidic technology are valuable assets for advancing individualized medicine plans such as new treatment protocols and diagnosis approaches. Individualized medicine research has been recently explored for applications such as point-of-care testing and individualized drug therapy. By carefully fusing microfluidic technology into these applications, we would be able to improve the effectiveness in detecting biomolecules and monitoring drug delivery profiles in vivo. In this review, we report and discuss the recent development, advancement, and future trends of using microfluidic technology for individualized diagnosis and therapy studies in vitro and in vivo.

 Please wait while we load your content...
Please wait while we load your content...