Cu(OAc)2·H2O/NH2NH2·H2O: an efficient catalyst system that in situ generates Cu2O nanoparticles and HOAc for Huisgen click reactions†
Abstract
A combination of Cu(OAc)2·H2O and NH2NH2·H2O, which in situ generates Cu2O-NPs and HOAc in water at room temperature, has been developed as a highly efficient catalytic system for Huisgen click reactions. Our results indicate that the in situ generated Cu2O-NPs and HOAc play important roles in the Huisgen click reaction.
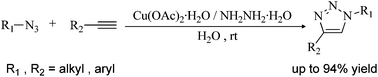

 Please wait while we load your content...
Please wait while we load your content...