Gadolinium-loaded polychelating amphiphilic polymer as an enhanced MRI contrast agent for human multiple myeloma and non Hodgkin's lymphoma (human Burkitt's lymphoma)†
Dorota Kozlowskaa,
Swati Biswasb,
Eoin K. Foxc,
Bing Wuc,
Ferdia Bolsterd,
Om Prakash Edupugantia,
Vladimir Torchilinb,
Stephen Eustaced,
Mauro Bottae,
Richard O'Kennedya and
Dermot F. Brougham*c
aSchool of Biotechnology, Dublin City University, Dublin 9, Ireland
bPharmaceutical Biotechnology and Nanomedicine, Northeastern University, Boston, MA 02115, USA
cSchool of Chemical Sciences, National Institute for Cellular Biotechnology, Dublin City University, Dublin 9, Ireland. E-mail: dermot.brougham@dcu.ie
dRadiology Department, Mater Misericordiae University Hospital, Eccles Street, Dublin 7, Ireland
eDipartimento di Scienze e Innovazione Tecnologica, Università del Piemonte Orientale “Amedeo Avogadro”, Viale T. Michel 11, 15121, Alessandria, Italy
First published on 1st April 2014
Abstract
Liposomes, loaded with gadolinium (Gd) ions using different membrane-incorporated chelating lipids and functionalized with monoclonal anti-CD138 (syndecan-1) antibody were prepared. Nuclear Magnetic Resonance Dispersion (NMRD) analysis showed that use of the polychelating amphiphilic polymer (PAP) increases both the Gd content and the spin–lattice relaxivity of the Gd-loaded-PAP–liposomes as compared to Gd–DTPA–BSA equivalents. The potential application of contrast syndecan-1– and Rituximab–liposomes, for application as a novel minimally invasive diagnostic agent for multiple myeloma and non Hodgkin's lymphoma was investigated.
Introduction
Magnetic resonance imaging (MR) has become one of the leading non-invasive modalities used clinically. It is widely applied to obtain images of pathological tissue, as it is capable of generating three-dimensional non-invasive images of opaque tissues at near cellular resolution and may have the greatest potential to exploit the possibilities that molecular imaging presents. MRI exhibits good spatial resolution but low sensitivity in the region of 10−3 to 10−5 mol L−1. This low sensitivity can be overcome by using contrast agents (CA) with high relaxivity. Although MRI is a non-invasive technique around 25% of the current MR-examinations include a contrast agent. These agents consist of molecules which incorporate a paramagnetic metal ion, most commonly gadolinium or iron. The inclusion of a paramagnetic agent, such as Gd, increases the R1 and R2 relaxation rates. However, the best visualization is obtained using T1-weighted images because in tissue the percentage change in R1 is much greater than in R2. Gd is an example of paramagnetic agent which directly affects water protons in its close vicinity. Hence, the influence of paramagnetic agents is very local and they should ideally be in contact with water with adequate exchange. In contrast, superparamagnetic agents (i.e. Fe) affect the magnetic field independently of their environment and, thus, their influence in terms of contrast extends well beyond their immediate surroundings.1To enhance the MR-efficacy and/or to improve the site-specific delivery of gadolinium (Gd) based MR-contrast agents, various systems such as nanoparticles have been suggested as carriers.2 Nanoparticles, including liposomes and other nanoscale constructs (solid lipid nanoparticles, polymeric nanoparticles, nanocrystals, dendrimers and carbon nanotubes) are the focus of intense research, due to potential applications in the biomedical, optical, and electronic fields.3 In this research liposomes were generated to target human multiple myeloma and human Burkitt's lymphoma for diagnostic applications.
Human multiple myeloma (MM) is a plasma B cell malignancy, which occurs in bone marrow. MM accounts for slightly more than 10% of all haematological malignancies.4 CD138 (syndecan-1), is over-expressed on the major types of human multiple myeloma cancer cells in bone marrow and is a good marker of this malignancy.5
Lymphoma is a broad term that encompasses a wide group of malignancies of the lymphoproliferative system of different biology and prognosis. Lymphoma is generally subdivided into two main groups, Hodgkins Lymphoma (HL) and Non-Hodgkins Lymphoma (NHL). NHL is the most common primary haematological malignancy and its incidence is on the rise. Greater than 90% of all NHL are of B-cell origin. At present surgical excision of a lymph node followed by histological and immunohistochemical examination of the lymph node is the gold standard for diagnosing lymphoma. CD20 is an antigen expressed specifically by B cells, and is homogeneously expressed on more than 90% of B cell lymphomas at a density of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 200
000 to 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 molecules per lymphoma cell.6
000 molecules per lymphoma cell.6
Targeting of MRI contrast agents to distinct molecules associated with tissue pathology could enable more specific diagnosis by MRI leading to characterisation of the disease at the molecular level in vivo. Qiao et al.7 demonstrated that a Gd3+-chelated protein-based (ProCA1) MRI agent, which was HER2-targeted antibody-conjugated, showed higher relaxivity than the non-targeted equivalent in ovarian (SKOV-3) and breast (MDA-MB-231) cancer cell lines in vivo. Furthermore, Wang et al.8 prepared novel anti-BRCAA1 (a protein over-expressed in 64% of gastric cancer tissue) monoclonal antibodies that were linked to fluorescent magnetic nanoparticles (FMNPs) composed of silica-coated quantum dots and super-paramagnetic nanoparticles. They were effective for in vivo dual modal imaging by fluorescence and magnetic resonance.
Antibody-labelled composition-optimised liposome-encapsulated gadolinium have significantly improved kinetics due to longer blood circulation, selective targeting/distribution to tumors relative to healthy tissues, improved extravasation from blood at the tumor's location and enhanced tumour binding due to specific antibody docking.9
Targeted Gd-loaded nanoparticles can overcome specificity problems related to non-targeted paramagnetic agents. Moreover, an amplification strategy for gadolinium-containing contrast agents is required to enhance sensitivity. Gadolinium-containing contrast agents currently available require a tissue concentration in the order of 10−7 mol g−1 to obtain sufficient contrast in the resulting image.1,10 This level is too high to facilitate imaging of sparse molecular biomarkers expressed on cells since such molecules are normally present in low concentrations of 10−9 to 10−13 mol g−1.11 One solution is to use nanoparticles since a high payload of contrast agent can be incorporated per single particle, thus increasing the effective relaxivity per particle, and, consequently, the signal detected during imaging increases.12 PAP (polychelating amphiphilic polymer) chelates many contrast agents per molecule and is capable of increasing relaxivity per particle. PAP was previously synthesized and used for tumor-targeting of PEGylated 2C5-conjugated-liposomes in vitro13 and in vivo.14
The long term goal of this research was the generation of a novel contrast agent for minimally invasive diagnosis of both human multiple myeloma and Burkitt's lymphoma in humans. In the reported study, Gd-loaded PAP-containing PEGylated-liposomes were evaluated as potential tumor-targeted contrast agents for MRI. The T1 relaxation time of these liposomes was compared with Gd–DTPA–BSA-loaded liposomes and it was demonstrated that Gd-loaded-PAP–liposomes show higher r1 relaxivity than Gd–DTPA–BSA (diethylenetriaminepenta-acetic acid α,ω-bis(8-stearoylamido-2,6-dioxaoctylamide)gadolinium salt)-liposomes loaded with monomeric Gd–DTPA, at the same molar ratio as the chelate. It was also shown that targeted anti-CD138-conjugated-Gd–PAP–liposomes bound significantly more to human multiple myeloma cell lines compared to plain contrast liposomes (blank liposomes without antibody) or negative control antibody-conjugated-Gd–PAP–liposomes. These results can be considered as an important step in the development of targeted contrast agents for minimally invasive diagnosis of MM and Burkitt's lymphoma.
The paramagnetic gadolinium, Gd3+, ion has been long used to overcome sensitivity issues related to MR imaging as its presence shortens the T1 and T2 relaxation times of the surrounding water.19 It has been found that liposome-based contrast agents, including Gd ions, demonstrate potentially promising results for use as diagnostic imaging agents for different types of cancer.14,17,20–22 The efficacy of the Gd3+ – bearing species for altering the relaxation properties of the water 1H nuclei can be quantified by the relaxivities, r1 or r2, which are defined as the change in R1 or R2 relaxation rates, per unit concentration of the contrast agent; r1 and r2 have units of s−1 mM−1.
The traditional method of encapsulating mainly water soluble contrast agents, used Gd3+ chelates, within the liposome lumen and has the disadvantage that the entrapped agents easily ‘leak out’ before the agent can reach the target site. This problem can be avoided by chelation of Gd3+ with lipid-compatible molecules like DTPA–BSA, DOTA–DSA or NGPE–PLL–DTPA that readily insert their chains into the membrane bilayer. In this way, Gd3+ can be anchored to the liposome surface instead of being encapsulated in the lumen. Kamaly et al. found that a liposomal formulation containing Gd–DOTA–DSA was non-toxic to HeLa cells, as demonstrated by the LDH viability assay.23 Hence we prepared liposomes using Gd-loaded NGPE–PLL–DTPA (PAP) and Gd-loaded DTPA–BSA, to anchor the contrast agent to the liposome bilayer whilst hopefully avoiding toxic effects on cells. These complexes are particularly advantageous as a high payload of ions can be incorporated in a single liposome, thus increasing the effective relaxation rate enhancement achievable.24 Previously Torchilin et al. showed that the NGPE–PLL-CBZ complex generates up to 11 amine groups that are available to undergo reaction with DTPA, which can subsequently be loaded with Gd ions25 with a resulting average loading of 6 ions. Erdogan et al. (2006) demonstrated that liposomes loaded with Gd via membrane-incorporated polychelating amphiphilic polymer (PAP) significantly increased the Gd content and relaxation rate, R1, of PEGylated liposomes.13
Materials and methods
Materials
L-α-Phosphatidylcholine, hydrogenated (egg, chicken) (egg PC), cholesterol, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[carboxy(polyethylene glycol)2000]-carboxylic acid (PEG–DSPE–carboxylic acid), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE–PEG-methoxy), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(glutaryl) (sodium salt) (NGPE), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(carboxyfluorescein) (FITC–PE), polycarbonate membranes (‘cut-off’ 100 nm) were purchased from Avanti Polar Lipids, Inc. (Delfzyl, Netherlands). Poly-ε-CBZ-DL-lysine (MW 5500) (PLL-CBZ), N,N′-dicyclohexyldiimide (DCC), N-hydroxysuccinimide (NHS), GdCl3·6H2O, succinic anhydride, dimethylformamide (DMF) and penicillin–streptomycin were obtained from Sigma-Aldrich (Hannover, Germany). Vivaspin columns (5000 Da) were supplied by Sartorius (Goettingen, Germany).Chemicals used to prepare buffer solutions were analytical grade.
RPMI 1640 medium and foetal bovine serum (FBS) were obtained from Bio-sciences (Crofton Road, Dun Laoghaire, Co. Dublin, Ireland).
Diethylenetriaminepentaacetic acid α,ω-bis(8-stearoylamido-3,6-dioxaoctylamide)gadolinium salt (Gd–DTPA–BSA monohydrate) was purchased from Azopharma (St. Louis, MO, USA).
U266 and RPMI8226 human multiple myeloma were supplied by Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Braunschweig, Germany). The RAMOS, L428 Hodgkin's disease-derived cell line was kindly donated by Dr Dermot Walls, School of Biotechnology, Dublin City University, Ireland.
Methods
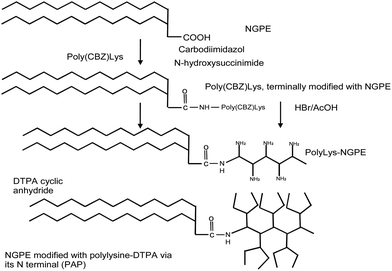
All NH2 groups of PLL-CBZ were protected by a CBZ group, except one which was linked to the activated carboxylic group of NGPE. To perform this reaction, 186 mg of PLL-CBZ was dissolved in 4 mL of DMF solvent and pooled together with activated NGPE phospholipids. The reaction was performed for 12 hours at room temperature with stirring. Chloroform was then evaporated from the mixture and product was precipitated using distilled water. The NGPE–PLL-CBZ product was freeze-dried for 48 hours and 193 mg of the NGPE–PLL-CBZ compound obtained. The NGPE–PLL-CBZ complex was de-protected from the CBZ group using 2 mL of 33% (v/v) hydrogen bromide in acetic acid for 2 hours at room temperature with stirring. The complex was precipitated using diethyl ether and freeze-dried for 12 hours. De-protected complex of NGPE–PLL (107 mg) was obtained. De-protection of the NGPE–PLL-CBZ complex generated a high number of NH2 groups which were available for reaction with DTPA. NGPE–PLL was treated with DTPA anhydride in the presence of triethylamine for 16 hours at room temperature with stirring. Succinic anhydride was added and the reaction was allowed to proceed for 1 hour at room temperature. The complex was then dialysed against distilled water (‘cut-off’ 3500 kDa) to remove unbound succinic anhydride (Fig. 1).
The gadolinium content in contrast liposomes (Gd–DTPA–BSA–liposomes and Gd–PAP–liposomes) was then determined using Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES). For ICP-AES analyses, liposomes were dissolved in 0.2% (v/v) Triton X-100 and made up quantitatively for analysis. A linear calibration curve, with R2 > 0.99, for GdCl3 standards in the same concentration ranges was established, and the gadolinium content of the liposome suspensions determined. Spin–lattice relaxivity, r1, values were then obtained by normalizing the measured rates for the gadolinium content, according to:
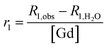 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mobile phase on a silica gel plate (60 Å pore diameter). The dried silica plate was placed in an iodine tank for visualization of the separated lipid spots.
1) mobile phase on a silica gel plate (60 Å pore diameter). The dried silica plate was placed in an iodine tank for visualization of the separated lipid spots.Anti-CD138 (syndecan-1) monoclonal antibody (0.2 mg) was at a 40 molar excess of esterified DSPE–PEG–COOH lipid generating DSPE–PEG–CO–NH–anti-CD138-antibody. A co-incubation method was used to prepare anti-CD138-conjugated liposomes.13,14,17,18
To prepare contrast immunoliposomes, 62.25 mol% egg PC, 30 mol% cholesterol, 5 mol% PEG2000–PE, 0.5 mol% FITC–PE and 1.75 mol% Gd–PAP were used. Solvent was evaporated under nitrogen. The lipid film was hydrated in 1 mL 0.1 M PBS, pH 7.3, by vortexing for 25 min at room temperature. Liposomes were then extruded through a 100 nm polycarbonate membrane. Then, 2 mol% of DSPE–PEG–CO–NH–anti-CD138-antibody previously prepared was co-incubated with liposomes by stirring overnight at 4 °C.
Plain Gd–PAP–liposomes (liposomes without antibody conjugated) and Herceptin®–Gd–PAP–liposomes were prepared as a negative control. Herceptin® (Trastuzumab) is a humanized monoclonal antibody which interferes with HER2 receptor overexpressed in breast cancer cells.
Separation of non-conjugated anti-CD138 antibodies from contrast anti-CD138-conjugated-liposomes was performed by dialysis against 0.1 M PBS, pH 7.3, for 2 days (‘cut-off’ 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da).
000 Da).
The Rituximab antibody (0.6 mg) was conjugated to 2 mol% of DSPE–PEG–COOH by firstly esterifying the DSPE–PEG–COOH using DCC and NHS. Rituximab is a chimeric monoclonal antibody that binds to CD20 which is primarily found on B cells.
A forty molar excess of this esterified lipid was conjugated to the Rituximab monoclonal antibody by incubation in 0.1 M PBS, pH 8.5, overnight at 4 °C. This Rituximab-conjugated-phospholipid solution was dialyzed using 3500 Da ‘cut-off’ dialysis tubing against 0.1 M PBS, pH 7.4, for 2 h to remove DMSO, in which esterified lipid was resuspended. Subsequently the mixture was co-incubated with the previously prepared liposomes at 4 °C overnight.
Fluorescently-labelled-liposomes were dialyzed against PBS, pH 7.4, (‘cut-off’ 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) for 2 days at 4 °C to remove unbound antibody.
000 Da) for 2 days at 4 °C to remove unbound antibody.
Binding of the Rituximab monoclonal antibody to RAMOS (human Burkitt's lymphoma) and L428 (Hodgkin's disease-derived) cells was also tested by flow cytometry. RAMOS and L428 cells (106) were incubated with 1 μg mL−1 of Rituximab or Avastin® antibody for 30 min at 4 °C. The cells were then washed with PBS, pH 7.4, and incubated with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 dilution of Cy5-labelled anti-human IgG secondary antibody for 30 min at 4 °C. Cells were washed three times with FACS buffer (0.1 M PBS, pH 7.4 with 2% (v/v) FBS and 0.05% (w/v) NaN3) and immediately analyzed on a FACS Calibur instrument.
2000 dilution of Cy5-labelled anti-human IgG secondary antibody for 30 min at 4 °C. Cells were washed three times with FACS buffer (0.1 M PBS, pH 7.4 with 2% (v/v) FBS and 0.05% (w/v) NaN3) and immediately analyzed on a FACS Calibur instrument.
Results and discussion
Synthesis and physical characterisation
The synthetic scheme for the preparation of Gd-loaded polychelating amphiphilic polymer (PAP) is shown in Fig. 1. This lipid provides the possibility of increasing the Gd-loading, as compared to lipidic carriers such as Gd–DTPA–BSA, which can accommodate only a single ion. In the current study, the mole fraction of the Gd–lipid was fixed at 1.75 mol%, for both types of lipid, as the report by Weissig et al. (2000) suggested that this is an optimal molar fraction for contrast liposome preparation.16 The size of contrast liposomes was determined to be 150.3 nm (PDI 0.18) for Gd–DTPA–BSA–liposomes and 120.4 nm (PDI 0.25) for Gd–PAP–liposomes, using PCS. Indeed, PCS shows that liposome suspensions of these two formulations were stable for at least 14 days, and, on exposure to strong magnetic fields. Zeta potential for Gd–DTPA–BSA was approximately −40 mV with variations in the 5–7 mV range. Elemental analysis, by ICP-AES showed a gadolinium concentration of 2.18 mM of in Gd–DTPA–BSA–liposomes and 4.46 mM in Gd–PAP–liposomes. This is equivalent to yields of 67 and 60% for Gd–DTPA–BSA– and Gd–PAP–liposomes, respectively, following all processing steps.The spin–lattice relaxivity r1 of Gd–PAP–liposomes was found to be 33 s−1 mM−1 (Gd concentration) compared to 15 s−1 mM−1 for Gd–DTPA–BSA–liposomes, at a measurement field of 60 MHz, which is within the clinical MRI range. Given that the Gd–PAP ligand typically contains 6 ions, this surprising result suggests that a single Gd–PAP ligand could potentially provide improved image contest, under T1-weighting, by a factor of ∼13 per lipid incorporated.
To further investigate the unusual magnetic resonance characteristics of the contrast liposomes NMRD profiles were recorded for the suspensions at 25 °C, as shown in Fig. 2. The profile for Gd–DTPA–BSA–liposomes is found to be similar to those for related gadolinium-containing suspensions. A low frequency plateau, at 13–14 s−1 mM−1, an r1 maximum at 15–25 MHz and a decrease in r1 into the clinical MRI range are very characteristic. These features have been reported in the profiles of several amphiphilic gadolinium chelates included in liposomal formulations. For instance, Gd–DMPE–DTPA with OPPC,26 or Gd–DTPA bisamides with DPPC.27 The profile of Gd–PAP–BSA–liposomes shows a significant increase in relaxivity at all frequencies, but in particular in the clinical range. It should be emphasised that the Gd–PAP– and Gd–DTPA–BSA–liposomes are of the same size and are formulated with the same fraction of Gd-bearing lipid.
The NMRD profiles have been fitted only in the high field region (>3 MHz) because of the well-known limitations of the Solomon–Bloembergen–Morgan (SBM) relaxation theory at low magnetic fields strengths under the slow motion regime.28 In order to separate the local molecular rotation of the chelates (characterized by a correlation time τRL) from the global tumbling motion of the nanoparticle (τRG), the Lipari–Szabo model free approach for the rotational dynamics has been incorporated into SBM theory.29 The best fit was obtained by fixing the number of coordinated water molecules (q = 1), their distance from the paramagnetic metal ion (rGdH = 3.0 Å), the values for the distance of closest approach of the outer sphere solvent molecules to Gd3+ (a = 4.0 Å) and for the relative diffusion coefficient of solute and solvent molecules (D = 2.24 × 10−5 cm2 s−1). The adjustable parameters used were the rate of water exchange (kex = 1/τM), the parameters describing the electronic relaxation times T1,2e (Δ2, τV), τRG and τRL, and the order parameter S2 which represents their degree of correlation (0 < S2 < 1). In addition, based on published data in the case of similar DTPA derivatives, the parameter kex was only allowed to vary in the range 1–10 × 106 s−1.28–31 Moreover, the parameters Δ2 and τV should only be regarded as empirical fitting parameters and do not assume a clear physical meaning for these slowly tumbling systems. The best-fit parameters (Table 1) indicate a pronounced difference between the global motion of the nanoparticle and the local rotation of the complex around the lipophilic chains for Gd–DTPA–BSA–liposomes.
| Parameter | Gd–DTPA–BSA | Gd–PAP |
|---|---|---|
| a At 40 MHz and per Gd. The error bars represent an estimate of the uncertainty in the parameters based on a sensitivity analysis of the fit to the data. | ||
| r1p/mM−1 s−1a | 17.1 ± 0.2 | 33.8 ± 0.3 |
| Δ2/1019 s−2 | 1.9 ± 0.2 | 2.7 ± 0.2 |
| τV/ps | 32 ± 3 | 9 ± 1 |
| τRL/ns | 0.70 ± 0.09 | 1.7 ± 0.2 |
| τRG/ns | 6.6 ± 4 | 6.6 ± 2 |
| S2 | 0.13 ± 0.03 | 0.33 ± 0.05 |
| 310kex/106 s−1 | 1.7 ± 0.3 | 10.0 ± 0.5 |
This high degree of rotational flexibility, revealed by the low value of S2, is associated with a slow rate of bound water exchange and both of these features represent a limitation to the relaxivity at imaging fields. This scenario, which was previously identified,32 is in line with the physical expectation for DTPA–bisamide derivatives. In this case, the slow rate of water exchange represents the dominant factor that limits the relaxivity at both low and high fields. The effectiveness of the liposomes as MRI probes is significantly enhanced by restricting rotational flexibility of the Gd-chelates incorporated in the membrane and, at the same time, increasing kex. The improved motional coupling imposed by the spatial restriction due to the coordination of multiple lanthanide ions on each ligand is reflected by higher relaxivity values for Gd–PAP–liposomes over the entire frequency range investigated. In fact, both τRL and the order parameter, S2, assume larger values in this case (Table 1). Furthermore, the faster exchange of the bound water molecules, typical of DTPA-monoamide derivatives, is an additional contributing factor to the increase in r1p of about 100% at 40 MHz.
It is also possible that, following extrusion and equilibration, the larger Gd–PAP ligand may be preferentially found on the outer leaflet due to steric considerations, so enhancing r1, relative to Gd–DTPA equivalents. This pre-supposes that water permeation across the membrane is limiting. In the case of limited diffusion of water molecules through the membrane, the relaxivity increases with temperature since the contribution to r1 of the paramagnetic centers localized in the inner leaflet also increases. In contrast, the relaxivity of both liposomal systems decreases with increasing temperature (see ESI†) and this suggests that their different distribution in the outer-and inner leaflets, if indeed it does occur, is not the main factor that explains their different relaxivity.
Antibody loading
The number of antibody units per liposome was estimated under the assumptions that the size of an immunoliposome was equal to the average hydrodynamic size measured by PCS; here we take an average value of 135 nm, and that the vesicles were unilamellar and spherical.Firstly, the number of lipid molecules in 1 mL of the final suspensions can be calculated knowing all volumes and assuming quantitative conversion of the original formulation of 62.25 mol% of egg PC (5.2 μmol), 30 mol% of cholesterol (2.5 μmol), 5 mol% of DSPE–PEG–methoxy (0.416 μmol), 1.75 mol% Gd–DTPA–BSA (0.156 μmol) and 0.5 mol% of FITC–PE (0.0416 μmol) to be ca. 5.3 × 1013 molecules per mL.33 The average lipid head group area (weighted by the mole fractions), was calculated to be 0.50 nm2, see Table 2. This gives an estimate of 212![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 578 lipids for each 135 nm liposome. Using this footprint and the liposome concentration we can also estimate that there are 1.52 × 1016 liposomes per mL.
578 lipids for each 135 nm liposome. Using this footprint and the liposome concentration we can also estimate that there are 1.52 × 1016 liposomes per mL.
| Lipid | Component | Area [nm2] |
|---|---|---|
| Egg PC | Head group | 0.55 |
| DSPE | Head group | 0.55 |
| PE | Head group | 0.55 |
| Cholesterol | Head group | 0.40 |
| DTPA | Head group | 0.70 |
The antibody (150 kDa) concentration in a solution of purified immunoliposomes was determined using the micro Lowry assay with Peterson's modification to be 103.18 μg mL−1, or 6.9 × 10−10 moles per mL of anti-CD138-conjugated-liposome solution, or ∼4.14 × 1017 antibody molecules. Hence we can estimate that there are on average ∼27 antibody molecules per liposome (=4.94 × 1017 antibody molecules/1.52 × 1016 liposomes).
The estimates of the numbers of molecules and liposomes are subject to the assumption of quantitative conversion of all the components in the formulation. However, that is not the case for the average number of ∼27 antibody molecules per liposome: we have determined the gadolinium incorporation to be 60%, by ICP-AES. The micro Lowry assay on the liposomes demonstrated 52% yield of antibody. Gravimetric analysis, for a range of lipid formulations prepared in our laboratory using the methods described, consistently gives yields of 60–70%.34 This demonstrates that the fractions of all the components incorporated are unchanged from the starting formulation, and the yield from the contrast immunoliposomes preparation process is in the range of 60% (mostly associated with extrusion). Hence it is reasonable to conclude that the number of antibody molecules per liposome is not significantly altered by the reduced yield after processing.
Finally, it was also determined that size of contrast liposomes increased after conjugation with anti-CD138 antibody. It was found that hydrodynamic diameter (from PCS) shifted from 158.6 nm to 161.7 nm for anti-CD138–Gd–DTPA–BSA–liposomes and from 193.9 nm to 208.8 nm for anti-CD138–Gd–PAP–liposomes. Assuming an antibody size of 18 nm, one would expect addition of ca. 27 antibodies to produce a marginal increase in hydrodynamic size. PCS is sensitive to the extent of hydration and is very sensitive to the larger liposomes in the size distribution, so it is not possible to confirm the number of conjugated antibodies by this technique. However, the observations are consistent with significant conjugation, as is shown by the functional study below.
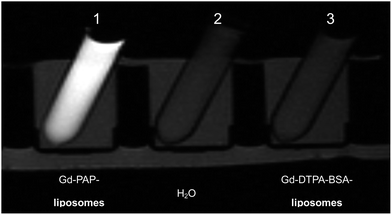
Phantoms of contrast Gd–DTPA–BSA– and Gd–PAP–liposomes were then assessed by T1-weighted MRI at 1.5 T (Fig. 3). Significant intensity enhancement is observed for Gd–PAP–liposomes as compared to Gd–DTPA–BSA–liposomes, at the same Gd–lipid concentration of 1.75 mol%. The signal of the Gd–DTPA–BSA–liposomes was similar to that obtained for pure water. This demonstrates the possibility of generating contrast under T1-weighting conditions in MRI. Phantoms of anti-CD138–Gd–DTPA–BSA- and anti-CD138–Gd–PAP-conjugated-liposomes were also imaged with T1-weighting. Again, it is apparent from Fig. 4, that the signal of anti-CD138–Gd–PAP-conjugated-liposomes is significantly enhanced relative to anti-CD138–Gd–DTPA–BSA-conjugated-liposomes, in this case at the same Gd concentration of 1.75 mol%. The signal generated by the anti-CD138–Gd–DTPA–BSA-conjugated-liposomes was similar to that given by the control (pure water). This MRI analysis demonstrates that significant contrast can be generated using both contrast liposomes and contrast immunoliposomes under imaging conditions and, hence, the presence of the antibody at an average of 27 units per liposome does not compromise the key physicochemical parameters determining the contrast efficiency of the targeted carriers.
Immuno-liposome performance
Subsequently, human multiple myeloma cancer cell lines (U266 and RPMI8226) were tested for CD138 protein expression just before the performance of in vitro studies using anti-CD138-contrast liposomes. It was estimated that U266 human multiple myeloma cancer cells express CD138 at a significantly (p < 0.05) higher level (approximately 26 fold) in comparison to RPMI8226 and L428 Hodgkin's disease-derived cell lines (Fig. 5). The Hodgkin's disease-derived L428 cell line does not express CD138. Human Burkitt's lymphoma (RAMOS) cells and L428 were tested for CD20 antigen expression with the Rituximab antibody. However, no binding was observed using 1 μg mL−1 of Rituximab antibody with the L428 cell line (Fig. 6). The RAMOS cell line was positively stained using these three different Rituximab concentrations. Thus, it was shown that the RAMOS cell line is CD20-positive and L428 is CD20-negative.Studies on in vitro interactions of contrast anti-CD138-conjugated liposomes with cancer cell lines were also performed and visualized by flow cytometry. Approximately 5 × 105 cells were incubated with 0.1 mg mL−1 of liposomes in ‘serum-free’ medium for 1–1.5 hours at 37 °C. Gd–PAP (1.75 mol%) was used in contrast anti-CD138-conjugated liposome preparation. The same amount of contrast lipid was used to generate unconjugated liposomes (liposomes without antibody attached) and as a negative control Herceptin®-conjugated-contrast liposomes. All preparations were washed three times with 0.1 M PBS, pH 7.3.
It was found that anti-CD138–Gd–PAP-conjugated-liposomes bound approximately 2.1 fold more strongly to U266 human multiple myeloma cells than did control liposomes (Herceptin®-conjugated-Gd–PAP–liposomes) (Fig. 7). This suggests that fluorescently-labelled contrast liposomes bind more significantly to U266 human multiple myeloma cell line compared to do negative control liposomes.
In vitro interactions of fluorescently-labelled Rituximab-conjugated-liposomes with human Burkitt's lymphoma were analysed using flow cytometry (Fig. 8). The RAMOS cell line bound Rituximab-conjugated-labelled-liposomes 2.5 times more strongly than the L428 cell line. The Student's t-test confirms a statistically significant difference (0.001 < p < 0.01) in fluorescence between Avastin®-conjugated-liposomes (negative control) and Rituximab-conjugated-liposomes bound to the RAMOS cell line. These findings demonstrate that Rituximab-labelled liposomes bind specifically to the RAMOS cell line.
Conclusions
We have shown that Gd–PAP–liposomes exhibit significantly increased spin–lattice relaxivity (per gadolinium ion) as compared to equivalent Gd–DTPA–BSA–liposomes. Given the increased number of ions per lipid, Gd–PAP–liposomes may provide strong contrast under T1-weighting conditions in MRI. The NMRD analysis identified the key physicochemical differences between the two vehicles that give rise to the enhanced relaxivity for Gd–PAP–liposomes. We further demonstrated that the T1-weighting capability is maintained on conjugation of the monoclonal anti-CD138 antibody to produce contrast immunoliposomes. It was found that anti-CD138–Gd–PAP-conjugated-liposomes bound approximately 2.1 fold strongly to U266 human multiple myeloma cells than did negative control liposomes of Herceptin®–Gd–PAP–conjugated-liposomes. It was also found that fluorescently labelled Rituximab-conjugated-liposomes bound approximately 2.5 fold more strongly to RAMOS human Burkitt's lymphoma than to the negative control. A preliminary confirmation of the possibility of labelling human multiple myeloma cells with the targeted agent was also undertaken. These findings should contribute towards the preparation of novel, minimally invasive contrast agents for diagnosis of human multiple myeloma and human Burkitt's lymphoma.Acknowledgements
This study was supported by the Irish Cancer Society, Dublin City University, the Mater Misericordiae University Hospital (BKS06EUS) and the EU PHARMATLANTIC grant. Part of this work was carried out in the Pharmaceutical Biotechnology and Nanomedicine Centre at Northeastern University, Boston, USA. This project has received funding from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 316973.References
- G. M. Lanza, P. M. Winter, S. D. Caruthers, A. M. Morawski, A. H. Schmieder, K. C. Crowder and S. A. Wickline, J. Nucl. Cardiol., 2004, 11, 733–743 CrossRef PubMed.
- Y. Okuhata, Adv. Drug Delivery Rev., 1999, 37, 121–137 CrossRef CAS.
- D. Kozlowska, P. Foran, P. MacMahon, M. J. Shelly, S. Eustace and R. O'Kennedy, Adv. Drug Delivery Rev., 2009, 61, 1402–1411 CrossRef CAS PubMed.
- R. A. Kyle and S. V. Rajkumar, N. Engl. J. Med., 2004, 351, 1860–1873 CrossRef CAS PubMed.
- R. A. Kyle, E. D. Remstein, T. M. Therneau, A. Dispenzieri, P. J. Kurtin, J. M. Hodnefield, D. R. Larson, M. F. Plevak, D. F. Jelinek, R. Fonseca, L. J. Melton and S. V. Rajkumar, N. Engl. J. Med., 2007, 356, 2582–2590 CrossRef CAS PubMed.
- P. J. Macardle and I. C. Nicholson, J. Biol. Regul. Homeostatic Agents, 2002, 16, 136–138 CAS.
- J. Qiao, S. L. Li, L. Wei, J. Jiang, R. Long, H. Mao, L. Wei, L. Wang, H. Yang, H. E. Grossniklaus, Z. R. Liu and J. J. Yang, PLoS One, 2011, 6, e18103 CAS.
- K. Wang, J. Ruan, Q. Qian, H. Song, C. Bao, X. Zhang, Y. Kong, C. Zhang, G. Hu, J. Ni and D. Cui, J. Nanobiotechnol., 2011, 9, 23 CrossRef CAS PubMed.
- F. Perche and V. P. Torchilin, Recent Trends in Multifunctional Liposomal Nanocarriers for Enhanced Tumor Targeting, J. Drug Delivery, 2013, 2013, 1–32 CrossRef PubMed.
- H. Gupta and R. Weissleder, Magn. Reson. Imag. Clin. N. Am., 1996, 4, 171–184 CAS.
- G. J. Strijkers, W. J. Mulder, R. B. van Heeswijk, P. M. Frederik, P. Bomans, P. C. Magusin and K. Nicolay, MAGMA, 2005, 18, 186–192 CrossRef CAS PubMed.
- C. Glogard, G. Stensrud, R. Hovland, S. L. Fossheim and J. Klaveness, Int. J. Pharm., 2002, 233, 131–140 CrossRef CAS.
- S. Erdogan, A. Roby, R. Sawant, J. Hurley and V. P. Torchilin, J. Liposome Res., 2006, 16, 45–55 CrossRef CAS PubMed.
- S. Erdogan, Z. O. Medarova, A. Roby, A. Moore and V. P. Torchilin, J. Magn. Reson. Imag., 2008, 27, 574–580 CrossRef PubMed.
- V. S. Trubetskoy, J. A. Cannillo, A. Milshtein, G. L. Wolf and V. P. Torchilin, J. Magn. Reson. Imag., 1995, 13, 31–37 CrossRef CAS.
- V. V. Weissig, J. Babich and V. P. Torchilin, Colloids Surf., B, 2000, 18, 293–299 CrossRef CAS.
- S. Erdogan, A. Roby and V. P. Torchilin, Mol. Pharm., 2006, 3, 525–530 CrossRef CAS PubMed.
- S. Erdogan and V. P. Torchilin, Methods Mol. Biol., 2010, 605, 321–334 CAS.
- S. Aime, W. Dastru, S. G. Crich, E. Gianolio and V. Mainero, Biopolymers, 2002, 66, 419–428 CrossRef CAS PubMed.
- W. T. Phillips and B. Goins, J. Liposome Res., 2002, 12, 71–80 CrossRef CAS PubMed.
- V. P. Torchilin, Pharm. Biotechnol., 2002, 54, 235–252 CAS.
- V. P. Torchilin, Curr. Pharm. Biotechnol., 2000, 1, 183–215 CAS.
- N. Kamaly, T. Kalber, A. Ahmad, M. H. Oliver, P. W. So, A. H. Herlihy, J. D. Bell, M. R. Jorgensen and A. D. Miller, Bioconjugate Chem., 2008, 19, 118–129 CrossRef CAS PubMed.
- S. N. Goldberg, G. D. Girnan, A. N. Lukyanov, M. Ahmed, W. L. Monsky, G. S. Gazelle, J. C. Huertas, K. E. Stuart, T. Jacobs, V. P. Torchillin, E. F. Halpern and J. B. Kruskal, Radiology, 2002, 222, 797–804 CrossRef PubMed.
- V. P. Torchilin, V. S. Trubetskoya, A. M. Milshteyna, J. Canilloa, G. L. Wolfa, M. I. Papisovb, A. A. Bogdanovb, J. Narulac, B. A. Khawc and V. Omelyanenko, Biochim. Biophys. Acta, 1994, 28, 45–58 CAS.
- F. Alhaique, I. Bertini, M. Fragai, M. Carafa, C. Luchinat and G. Parigi, Inorg. Chim. Acta, 2002, 331, 151–157 CrossRef CAS.
- S. Laurent, E. L. Vander, C. Thirifays and R. N. Muller, Eur. Biophys. J., 2008, 37, 1007–1014 CrossRef CAS PubMed.
- D. D. Castelli, E. Gianolio, S. G. Crich, E. Terreno and S. Aime, Coord. Chem. Rev., 2008, 252, 2424–2443 CrossRef PubMed.
- G. Lipari and A. Szabo, J. Am. Chem. Soc., 1982, 104, 4546–4559 CrossRef CAS.
- S. Laurent, E. L. Vander, C. Thirifays and R. N. Muller, Langmuir, 2008, 24, 4347–4351 CrossRef CAS PubMed.
- M. Botta and L. Tei, Eur. J. Inorg. Chem., 2012, 12, 1945–1960 CrossRef.
- F. Kielar, L. Tei, E. Terreno and M. Botta, J. Am. Chem. Soc., 2010, 132, 7836–7837 CrossRef CAS PubMed.
- ASTM Standard D, American Society for Testing and Materials, 1985, 4182–4187 Search PubMed.
- C. C. Fraenza, C. J. Meledandri, E. Anoardo and D. F. Brougham, ChemPhysChem, 2014, 3, 423–435 Search PubMed; J. Perlo, C. J. Meledandri, E. Anoardo and D. F. Brougham, J. Phys. Chem. B, 2011, 115, 3444–3451 CrossRef CAS PubMed.
- D. D. Lasic, Liposomes in Gene Delivery, 1994, pp. 259–261 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra45400b |
| This journal is © The Royal Society of Chemistry 2014 |