Fabrication of Ag-coated AgBr nanoparticles and their plasmonic photocatalytic applications†
Jooyoung Song,
Inkyu Lee,
Jongmin Roh and
Jyongsik Jang*
WCU program of Chemical Convergence for Energy and Environment (C2E2), School of Chemical and Biological Engineering, College of Engineering, Seoul National University, 599 Gwanak-ro, Gwanak-gu, Seoul 151-742, Korea. E-mail: jsjang@plaza.snu.ac.kr; Fax: +82 2-888-1604; Tel: +82 2-880-7069
First published on 5th December 2013
Abstract
Ag-coated AgBr nanoparticles (Ag@AgBr) were fabricated via an aqueous, one-pot route. In this system, poly(vinyl alcohol) (PVA) acted as a stabilizer for the formation of nanoscale AgBr composites through interaction with Ag ions and their hydroxyl (–OH) groups. The mild reducing agent L-arginine was used for partial reduction of AgBr to form metallic Ag nanoparticles on the AgBr surface. The metallic Ag nanoparticles enhanced light absorption in the visible region due to surface plasmon resonance (SPR). The size of the synthesized nanoparticles was controlled by varying the reaction temperature, and was found to influence the light absorption of Ag@AgBr nanocomposites. The prepared nano-photocatalysts exhibited excellent photocatalytic activities under both visible light and direct sunlight.
Introduction
Recently, silver nanoparticle/silver halide (Ag@AgX; X = Cl, Br) composites have attracted great interest due to their excellent plasmonic photocatalytic activity under visible-light irradiation.1–9 The Ag nanoparticles act as visible-light sensitizers to enable effective photocatalysis based on their surface plasmon resonance (SPR) properties.10–12 Additionally, Ag nanoparticles on the surface of a semiconductor facilitate the separation of photo-generated electron–hole pairs by trapping electrons, leading to enhancement of photocatalytic efficiency.13,14 Among the various semiconductors, AgBr, which has a band gap of 2.6 eV, has been demonstrated to be an effective photocatalyst under visible light.15,16 For example, Wang et al.16 demonstrated that Ag@AgBr composites prepared by using ion-exchange decompose organic dyes under visible light. Ag@AgBr absorbs over a wide visible-light range because of the SPR of the deposited Ag nanoparticles. Jiang et al.9 synthesized indoor daylight active Ag@AgBr photocatalysts via a one-pot, microwave-assisted, non-aqueous method.In general, nano-photocatalysts increase catalytic efficiency due to their large surface areas. However, limited information about the fabrication of nanoscale AgX exists because of the fast precipitation reaction of Ag+ and Cl− (or Br−) ions. Therefore, development of reliable synthetic strategies for Ag@AgX nanostructures is needed. Solution synthesis has long been a preferred method for fabrication of metal nanostructures due to the potential for mass production at low cost.17,18 In the solution synthesis of metal nanostructures, polymers with metal-binding functional groups have been used as steric stabilizers or capping agents to reduce agglomeration and guide the shape of the nanostructures.19 In particular, polyvinylpyrrolidone (PVP) has been frequently used in the synthesis of metal nanostructures, including both AgCl:Ag7,20,21 and Ag/AgBr22 nanocomposites. For example, Sun et al.20 reported that PVP with hydroxyl (–OH) end groups can act as a surfactant in the synthesis of AgCl:Ag nanophotocatalysts. Poly(vinyl alcohol) (PVA) with –OH side groups has also been applied as a stabilizing agent for preparing various nanomaterials.23–25 In our previous work, we produced Ag@AgCl plasmonic nano-photocatalysts using PVA as a polymeric stabilizer.25 The lone electron pairs in the PVA interacted with Ag ions and provided reactive sites for the formation of AgCl nanoparticles.
Herein, we describe the synthesis and characterization of a plasmonic photocatalyst, Ag@AgBr, with greater catalytic efficiency than Ag@AgCl under visible light.9 PVA with –OH side groups was used as a stabilizer for synthesis of AgBr nanoparticles. The nanoparticles exhibited outstanding light absorption properties in the visible region as well as photocatalytic activity after partial reduction. The relationship between the particle size and the light absorption region of Ag@AgBr nanocomposites was systematically investigated, and their plasmonic photocatalytic activities were studied using dye-decomposition testing.
Methods
Materials
Silver nitrate (AgNO3, ≥99.0%) and sodium bromide (NaBr, ≥99.0%) were purchased from Aldrich (St Louis, MO) and used as precursor for the silver bromide nanoparticles. Poly(vinyl alcohol) (PVA) (Mw: 146![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–186
000–186![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and L-arginine (≥98%) were also purchased from Aldrich and used as stabilizer and reducing agent, respectively. For photocatalytic test, methylene blue (MB, ≥82.0%) was purchased from the Aldrich and used as organic dyes.
000) and L-arginine (≥98%) were also purchased from Aldrich and used as stabilizer and reducing agent, respectively. For photocatalytic test, methylene blue (MB, ≥82.0%) was purchased from the Aldrich and used as organic dyes.
Synthesis of the silver–silver bromide nanostructures
First, the AgBr nanoparticles were synthesized in the PVA dissolved aqueous solution. As a precursor, AgNO3 (0.059 mmol) was added to the PVA solution and the 1 mL of NaBr solution (58.8 mM) was sequentially injected dropwise. The reaction proceeded at various temperatures from 25 to 60 °C with magnetic stirring. After 30 min, the 1 mL of L-arginine dissolved solution (287 mM) was added to the reaction medium at 25 °C for the partial reduction of Ag+ of AgBr. After the 45 min of reduction process, the synthesized Ag@AgBr nanoparticles were centrifuged and washed with deionized water to remove residual reagents.Photocatalytic performance
The photocatalytic properties of the Ag@AgBr nanoparticles were evaluated in terms of the decomposition of dye molecules. The MB was used as organic dyes. For the test, 10 mg of as-prepared sample was added into the 50 mL of dye-dissolved aqueous solution (50 mg L−1). Prior to the light irradiation, the prepared solution was stirred for 30 min in the dark to ensure the establishment of the adsorption–desorption equilibrium for the dye. The photocatalytic test was performed under visible light irradiation by using a xenon-lamp (100 W) with a UV cut-off filter (λ > 400 nm). After specific contact time, about 3 mL aliquots were taken and centrifuged to remove the dispersed photocatalyst. The concentration of the dye was measured using the UV-vis spectroscopy. For practical application, the photocatalytic tests also performed under sunlight irradiation. The test proceeded from 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 to 15
00 to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 on a sunny day in June at latitude 37.6° North and longitude 127° East (Seoul City, Korea).
00 on a sunny day in June at latitude 37.6° North and longitude 127° East (Seoul City, Korea).
Instrumentation
Field-emission scanning electron microscopy (FE-SEM) images were obtained using a JEOL 6700 at an acceleration voltage of 10 kV and Energy dispersive X-ray (EDX) analysis was performed using INCA energy (Oxford Instruments Analytical Ltd., UK) coupled with an EDX facility. Ultraviolet-visible (UV-vis) spectra were obtained at 25 °C with a PerkinElmer Lambda-35 spectrometer and X-ray diffraction (XRD) analysis was performed with a Rigaku Model SmartLab. X-ray photoelectron spectroscopy (XPS) data was obtained using Sigma Probe electron spectroscope.Results and discussion
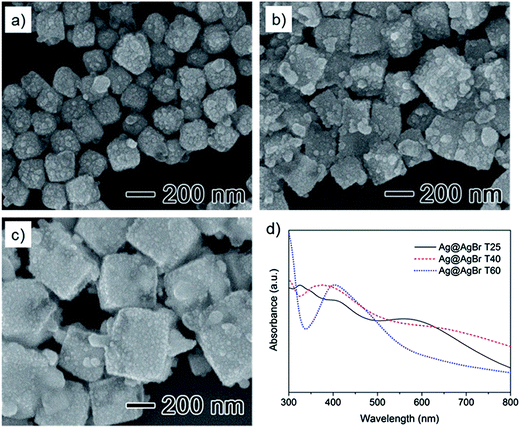
AgBr nanoparticles were synthesized via precipitation of Ag+ and Br− precursors. In general, because the precipitation reaction is very fast, the use of a stabilizer is necessary for size control of AgBr at the nanoscale. As briefly discussed in a previous study, PVA can serve as stabilizer for the formation of AgCl nanoparticles.25 Similarly, AgBr nanoparticles can be obtained using the PVA stabilization system with NaBr as the halide donor instead of NaCl. PVA forms a complex with Ag+ ions in aqueous solution because of its –OH side groups. When the Br− donor is added, AgBr nanoparticles are formed at the Ag+ reactive sites of the complex.Fig. 1 presents field-emission scanning electron microscopy (FE-SEM) images and the ultraviolet-visible (UV-vis) spectra of three as-prepared AgBr samples that were synthesized at different reaction temperature (25, 40, and 60 °C) using PVA with a molecular weight of 146![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–186
000–186![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and a molar ratio of PVA to Ag precursor of 100. The inset images at higher magnifications show that the synthesized AgBr exhibited cube-shaped morphology (Fig. 1a–c). When the reaction was performed at room temperature, the FE-SEM image indicated that the nanocubes had an edge length of ∼72 nm without any significant agglomeration (Fig. S1†). The edge length of the AgBr nanocubes was increased from ∼72 to ∼183 nm with increasing reaction temperature (from 25 to 60 °C). In addition, the size distribution of the resulting AgBr also increased with the reaction temperature. The mobility of the polymeric chain and the degree of freedom of the Ag+ and Br− ions increased with temperature.23 Consequently, the size of the AgBr nanocubes could be controlled by varying the reaction temperature. As shown in the UV-vis spectra (Fig. 1d), AgBr did not exhibit significant absorption in the visible-light region. Instead, shoulder peaks at around 315 nm were observed, which are consistent with previously reported observations for AgBr nanoparticles.26 The absorbance increased over the entire visible-light region as the size of the AgBr increased (from ∼72 to ∼183 nm) because larger particles scattered more incident light than smaller ones. When the reaction was performed in the absence of PVA stabilizer, agglomerated microscale precipitates with irregular shapes were obtained (Fig. S2†). Additionally, as the PVA content decreased, the nanoparticles lost their cubic structure, and the size-distribution was broadened (Fig. S3†). These results demonstrate that PVA plays a pivotal role in the formation of AgBr nanocubes during precipitation.
000 and a molar ratio of PVA to Ag precursor of 100. The inset images at higher magnifications show that the synthesized AgBr exhibited cube-shaped morphology (Fig. 1a–c). When the reaction was performed at room temperature, the FE-SEM image indicated that the nanocubes had an edge length of ∼72 nm without any significant agglomeration (Fig. S1†). The edge length of the AgBr nanocubes was increased from ∼72 to ∼183 nm with increasing reaction temperature (from 25 to 60 °C). In addition, the size distribution of the resulting AgBr also increased with the reaction temperature. The mobility of the polymeric chain and the degree of freedom of the Ag+ and Br− ions increased with temperature.23 Consequently, the size of the AgBr nanocubes could be controlled by varying the reaction temperature. As shown in the UV-vis spectra (Fig. 1d), AgBr did not exhibit significant absorption in the visible-light region. Instead, shoulder peaks at around 315 nm were observed, which are consistent with previously reported observations for AgBr nanoparticles.26 The absorbance increased over the entire visible-light region as the size of the AgBr increased (from ∼72 to ∼183 nm) because larger particles scattered more incident light than smaller ones. When the reaction was performed in the absence of PVA stabilizer, agglomerated microscale precipitates with irregular shapes were obtained (Fig. S2†). Additionally, as the PVA content decreased, the nanoparticles lost their cubic structure, and the size-distribution was broadened (Fig. S3†). These results demonstrate that PVA plays a pivotal role in the formation of AgBr nanocubes during precipitation.
The as-prepared AgBr nanoparticles were partially reduced by L-arginine at 25 °C under laboratory light conditions, resulting in the formation of Ag@AgBr nanocomposites. The X-ray diffraction (XRD) pattern of prepared Ag@AgBr nanoparticles (Fig. 2) featured additional peaks at 2θ = 38.0 compared with pristine AgBr nanocubes. This 2θ peak matched well with standard peaks for metallic Ag, thereby indicating that metallic Ag nanoparticles were formed on the AgBr surface after partial reduction.27,28 The extent of AgBr reduction was determined by energy-dispersive X-ray spectroscopy (EDX) analysis (Table 1). After reduction, the atomic percentage of Ag increased, and that of Br decreased, because the AgBr provided the precursor for metallic Ag during the reduction. The elemental composition and chemical states of Ag@AgBr samples were further verified by X-ray photoelectron spectroscopy (XPS); Ag, Br, C, and O species were detected (Fig. 3). The C and O contents of the Ag@AgBr samples were attributed to residual PVA stabilizer. The high-resolution Br 3d and Ag 3d spectra of a representative Ag@AgBr T25 sample are shown in Fig. 3b and c, respectively. The Ag@AgBr sample had binding energies of 67.7 and 68.8 eV, corresponding to Br 3d5/2 and Br 3d3/2, respectively; these spectral results are in agreement with reported values for AgBr.28,29 The Ag 3d spectrum of the Ag@AgBr T25 sample consisted of two peaks at 367 and 373 eV, corresponding to the binding energies of Ag 3d5/2 and Ag 3d3/2, respectively.28,29 These two peaks were further de-convoluted into two peaks at 366.8 and 372.8 eV associated with the Ag+ ion of AgBr, and two peaks at 368.5 and 374.5 eV associated with metallic Ag0.28,29 Based on the de-convoluted peaks, the Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AgBr ratio was calculated to be 1
AgBr ratio was calculated to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.2 (0.10), which is similar to the ratio value obtained using EDX analysis. The Ag
10.2 (0.10), which is similar to the ratio value obtained using EDX analysis. The Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AgBr ratio could be tailored by varying the reduction time. As shown in Fig. 4a–c, after partial reduction, nano-nodules (metallic Ag) were grown on the surface of the AgBr nanocubes. Additionally, the size of the Ag@AgBr nanoparticles increased from ∼72, ∼134, and ∼183 nm to ∼176, ∼240, and ∼370 nm, respectively (with a deviation of ∼10%) after reduction. Ostwald ripening occurred during the reaction due to the non-uniform size distribution of the AgBr substrates.30 Transmission electron microscopy (TEM) images could not be clearly obtained because exposing the Ag@AgBr particles to an electron beam with high current density caused reduction of the AgBr to metallic Ag.20 The color of the solutions after reduction were dark purple, purple, and salmon pink for Ag@AgBr T25, Ag@AgBr T40, and Ag@AgBr T60 samples. As shown in Fig. 4d, the synthesized Ag@AgBr nanocomposites exhibited distinct absorption peaks in the visible-light region compared with pristine AgBr nanocubes; these distinct absorption peaks were attributed to the SPR of the formed Ag nanoparticles. Each sample exhibited a strong absorption peak around ∼400 nm, which corresponded to the typical plasmon peak of Ag nanoparticles.31,32 The plasmon peak broadened with increasing size of the AgBr substrate because larger-sized AgBr substrates featured Ag nanoparticles with more diverse shapes and diameters. The Ag@AgBr nanocomposites exhibited a red-shifted absorption peak, which originated from the interaction between the AgBr substrate and the deposited metallic Ag nanoparticles. The Ag@AgBr T25 sample exhibited a unique peak at ∼600 nm, and the Ag@AgBr T40 sample exhibited a peak at ∼650 nm. The refractive index of a AgBr substrate results in shifting of the light absorption.33,34 For the Ag nanoparticles on the AgBr surface, the red-shifted absorption peak was observed because the refractive index of the AgBr (2.23) was higher than that of water (1.32) or air (1.0). In the case of the Ag@AgBr T60 sample, a red-shifted peak was not observed. Instead, absorption over the entire light region increased compared to the bare AgBr counterpart. This result was attributed to a large number of different shapes and sizes of Ag nanoparticles on the T60 sample, which caused plasmonic oscillations over a wide range of frequencies. Thus, the light absorption region of the Ag@AgBr nanocomposites was controlled by varying the size of the AgBr substrate.
AgBr ratio could be tailored by varying the reduction time. As shown in Fig. 4a–c, after partial reduction, nano-nodules (metallic Ag) were grown on the surface of the AgBr nanocubes. Additionally, the size of the Ag@AgBr nanoparticles increased from ∼72, ∼134, and ∼183 nm to ∼176, ∼240, and ∼370 nm, respectively (with a deviation of ∼10%) after reduction. Ostwald ripening occurred during the reaction due to the non-uniform size distribution of the AgBr substrates.30 Transmission electron microscopy (TEM) images could not be clearly obtained because exposing the Ag@AgBr particles to an electron beam with high current density caused reduction of the AgBr to metallic Ag.20 The color of the solutions after reduction were dark purple, purple, and salmon pink for Ag@AgBr T25, Ag@AgBr T40, and Ag@AgBr T60 samples. As shown in Fig. 4d, the synthesized Ag@AgBr nanocomposites exhibited distinct absorption peaks in the visible-light region compared with pristine AgBr nanocubes; these distinct absorption peaks were attributed to the SPR of the formed Ag nanoparticles. Each sample exhibited a strong absorption peak around ∼400 nm, which corresponded to the typical plasmon peak of Ag nanoparticles.31,32 The plasmon peak broadened with increasing size of the AgBr substrate because larger-sized AgBr substrates featured Ag nanoparticles with more diverse shapes and diameters. The Ag@AgBr nanocomposites exhibited a red-shifted absorption peak, which originated from the interaction between the AgBr substrate and the deposited metallic Ag nanoparticles. The Ag@AgBr T25 sample exhibited a unique peak at ∼600 nm, and the Ag@AgBr T40 sample exhibited a peak at ∼650 nm. The refractive index of a AgBr substrate results in shifting of the light absorption.33,34 For the Ag nanoparticles on the AgBr surface, the red-shifted absorption peak was observed because the refractive index of the AgBr (2.23) was higher than that of water (1.32) or air (1.0). In the case of the Ag@AgBr T60 sample, a red-shifted peak was not observed. Instead, absorption over the entire light region increased compared to the bare AgBr counterpart. This result was attributed to a large number of different shapes and sizes of Ag nanoparticles on the T60 sample, which caused plasmonic oscillations over a wide range of frequencies. Thus, the light absorption region of the Ag@AgBr nanocomposites was controlled by varying the size of the AgBr substrate.
| Sample | Atomic % of Ag | Atomic % of Br | Atomic ratio, Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AgBra AgBra |
Reduction % of Agb |
|---|---|---|---|---|
a Values of the atomic ratio of Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AgBr were calculated as atomic ratio of Ag AgBr were calculated as atomic ratio of Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AgBr = (A − B)/B (where A is the atomic % of Ag and B is the atomic % of Br).b Values of reduction % of Ag were calculated as reduction % of Ag = (A − B)/A. AgBr = (A − B)/B (where A is the atomic % of Ag and B is the atomic % of Br).b Values of reduction % of Ag were calculated as reduction % of Ag = (A − B)/A. |
||||
| Ag@AgBr T25 | 52.4 | 47.6 | 0.101 | 9.16 |
| Ag@AgBr T40 | 51.9 | 48.1 | 0.079 | 7.32 |
| Ag@AgBr T60 | 52.1 | 47.9 | 0.088 | 8.06 |
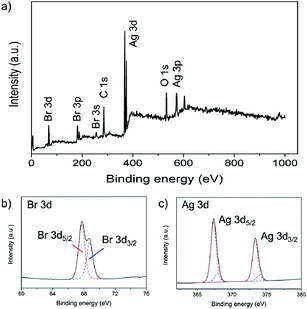 | ||
| Fig. 3 (a) XPS survey spectra of as-prepared Ag@AgBr. High-resolution XPS of (b) Br 3d and (c) Ag 3d spectra of the Ag@AgBr. | ||
The photocatalytic activities of the as-prepared Ag@AgBr nanoparticles were evaluated by decomposition of methylene-blue (MB) dye under visible light. Typically, 50 mg of sample is applied to degrade the dye molecules.35,36 However, in our study, because of their high surface area, 50 mg of the prepared nanophotocatalysts completely absorbed the MB molecules during the pre-adsorption step. Thus, we used 10 mg of sample for the photocatalytic activity tests. Fig. 5a shows the photocatalytic degradation of MB over the Ag@AgBr and bare AgBr under visible light (λ > 400 nm); in the figure, C0 is the concentration of MB at the adsorption–desorption equilibrium, and C is the concentration at a given time. Under our experimental conditions, bulk AgBr decomposed only 10% of MB after 30 min. The bare AgBr T25 sample exhibited some photocatalytic activity due to its enlarged surface area; it degraded 27.8% of MB after 30 min. The AgBr semiconductor had a bandgap of 2.6 eV, which could be excited under visible light.16 The loading of Ag nanoparticles on the AgBr surface remarkably enhanced the dye degradation efficiency of the samples. The Ag@AgBr T25 sample decomposed 95% of MB molecules after 30 min. Furthermore, the Ag@AgBr also showed decomposing activity against methyl orange (MO) and rhodamine B (Rh B) dyes (Fig S5†). Jiang et al.9 demonstrated that the interfacial interaction between metallic Ag and the underlying AgBr is critical to the photocatalytic activity of the Ag@AgBr composite. Under visible light, the intensity of electric fields in the vicinity of the Ag nanoparticles is increased due to their SPR, and the electric fields induce rapid formation of electron–hole pairs on the AgBr surface. In addition, the AgBr-based conventional semiconductor photocatalysis process simultaneously occurs because AgBr can be directly photo-excited under visible light to generate electron–hole pairs in its conduction and valence bands, respectively.9,37,38 Together with the injected SPR electrons from the Ag nanoparticles, the photogenerated electrons in the conduction band initiate catalysis.37,38 In the Ag@AgBr nanocomposite, both Ag nanoparticles and AgBr can respond to visible light, thereby producing more electrons and holes. Thus, Ag@AgBr exhibited higher photocatalytic activity than Ag@AgCl (Fig. S4†). The observed photocatalytic activity of the Ag@AgBr nanocomposites had the following order: T25 > T40 > T60. The photocatalytic activities increased with decreasing size of the Ag@AgBr nanocomposites. The smaller photocatalysts provided more sites for contacting and degrading the dye molecules, leading to enhanced photocatalytic activity. In addition, the SPR properties depended on the size, shape, and amount of the Ag nanoparticles.22,39 Thus, the Ag0 content and its size also influenced the photocatalytic efficiency of the Ag@AgBr nanocomposites. To mimic practical applications, an MB degradation test was performed on the Ag@AgBr under direct sunlight (air temperature of ∼30 °C). When the MB solution containing the synthesized Ag@AgBr T25 samples was placed under direct sunlight, the blue solution became purplish, the color of Ag@AgBr nanocomposites. Cycling tests were conducted to evaluate the stability of Ag@AgBr. After the MB was decomposed, the Ag@AgBr T25 samples were recovered by centrifugation and used in a new catalytic reaction. We determined that Ag@AgBr maintained its photocatalytic properties for three cycles (Fig. 5b). Fig. 5c plots the UV-vis absorption spectra of the test solution at different reaction times under sunlight irradiation. Decomposition of MB was complete within 20 min in the presence of Ag@AgBr T25 photocatalyst, which was much less time compared with that required under a 100 W xenon lamp. The enhanced activity under sunlight was attributed to the broadband nature of the sunlight, which includes UV wavelengths. Based on these results, synthesized Ag@AgBr nanocomposites can be utilized as visible-light-driven photocatalysts.
Conclusion
Plasmon photocatalytic Ag@AgBr nanocomposites were fabricated via a one-pot, environmentally benign route at a relatively mild temperature. PVA with –OH side groups was used as a stabilizer for the formation of nanoscale AgBr composites, and the size of the AgBr was controlled by varying the reaction temperature. In addition, the mild reducing agent L-arginine was used to induce the formation of metallic Ag nanoparticles on the surface of the AgBr substrates. The light absorption region of the synthesized Ag@AgBr nanocomposites was influenced by the size of the nanocomposite. Systematic photocatalytic testing of the prepared Ag@AgBr nanocomposites revealed that they exhibited greater photocatalytic activities than pristine AgBr under visible light due to the existence of metallic Ag on their surface; the photocatalytic efficiency of Ag@AgBr increased with decreasing nanoparticle size. Additionally, the Ag@AgBr nanocomposites exhibited excellent photocatalytic activity under sunlight irradiation.Acknowledgements
This research was supported by WCU (World Class University) program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (R31-10013).References
- P. Wang, B. Huang, X. Qin, X. Zhang, Y. Dai, J. Wei and M.-H. Whangbo, Angew. Chem., Int. Ed., 2008, 47, 7931 CrossRef CAS PubMed.
- Y. Li and Y. Ding, J. Phys. Chem. C, 2010, 114, 3175 CAS.
- H. Xu, H. Li, J. Xia, S. Yin, Z. Luo, L. Liu and L. Xu, ACS Appl. Mater. Interfaces, 2011, 3, 22 CAS.
- R. Dong, B. Tian, C. Zeng, T. Li, T. Wang and J. Zhang, J. Phys. Chem. C, 2013, 117, 213 CAS.
- Z. Lou, B. Huang, P. Wang, Z. Wang, X. Qin, X. Zhang, H. Cheng, Z. Zheng and Y. Dai, Dalton Trans., 2011, 40, 4104 RSC.
- P. Wang, B. Huang, Z. Lou, X. Zhang, X. Qin, Y. Dai, Z. Zheng and X. Wang, Chem.–Eur. J., 2010, 16, 538 CrossRef CAS PubMed.
- C. An, R. Wang, S. Wang and X. Zhang, J. Mater. Chem., 2011, 21, 11532 RSC.
- L. Dong, D. Liang and R. Gong, Eur. J. Inorg. Chem., 2012, 3200 CrossRef CAS.
- J. Jiang, H. Li and L. Zhang, Chem.–Eur. J., 2013, 18, 6360 CrossRef PubMed.
- B. Lim, Y. Xiong and Y. Xia, Angew. Chem., Int. Ed., 2007, 46, 9279 CrossRef CAS PubMed.
- K. A. Willets and R. P. Van Duyne, Annu. Rev. Phys. Chem., 2007, 58, 267 CrossRef CAS PubMed.
- P. Christopher, H. Xin and S. Linic, Nat. Chem., 2011, 3, 467 CAS.
- R. Georgekutty, M. K. Seery and S. C. Pillai, J. Phys. Chem. C, 2008, 112, 13563 CAS.
- A. Pan, L. Yao, Y. Qin, Y. Yang, D. Kim, R. Yu, B. Zou, P. Werner, M. Zacharias and U. Gosele, Nano Lett., 2008, 8, 3413 CrossRef CAS PubMed.
- S. Glaus and G. Calzaferri, Photochem. Photobiol. Sci., 2003, 2, 398 CAS.
- P. Wang, B. Huang, X. Zhang, X. Qin, H. Jin, Y. Dai, Z. Wang, J. Wei, J. Zhan, S. Wang, J. Wang and M.-H. Whangbo, Chem.–Eur. J., 2009, 15, 1821 CrossRef CAS PubMed.
- B. H. Jones and T. P. Lodge, J. Am. Chem. Soc., 2009, 131, 9746 CrossRef PubMed.
- L. Shi and C. Berkland, Adv. Mater., 2006, 18, 2315 CrossRef CAS.
- Y. Xia, Y. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., Int. Ed., 2009, 48, 60 CrossRef CAS PubMed.
- C. An, S. Peng and Y. Sun, Adv. Mater., 2010, 22, 2570 CrossRef CAS PubMed.
- L. Han, P. Wang, C. Zhu, Y. Zhai and S. Dong, Nanoscale, 2011, 3, 2931 RSC.
- Z. Wang, J. Liu and W. Chen, Dalton Trans., 2012, 41, 4866 RSC.
- J.-Y. Hong, H. Yoon and J. Jang, Small, 2010, 6, 679 CrossRef CAS PubMed.
- M. Choi, K.-H. Shin and J. Jang, J. Colloid Interface Sci., 2010, 341, 83 CrossRef CAS PubMed.
- J. Song, J. Roh, I. Lee and J. Jang, Dalton Trans., 2013, 42, 13897 RSC.
- H. Ohde, J. M. Rodriguez, X.-R. Ye and C. M. Wai, Chem. Commun., 2000, 2353 RSC.
- M. Zhu, P. Chen and M. Liu, Langmuir, 2012, 28, 3385 CrossRef CAS PubMed.
- C. An, J. Wang, W. Jiang, M. Zhang, X. Ming, S. Wang and Q. Zhang, Nanoscale, 2012, 4, 5646 RSC.
- C. An, J. Wang, C. Qin, W. Jiang, S. Wang, Y. Li and Q. Zhang, J. Mater. Chem., 2012, 22, 13153 RSC.
- S. Peng and Y. Sun, J. Mater. Chem., 2011, 21, 11644 RSC.
- J. Song, H. Kang, C. Lee, S. H. Hwang and J. Jang, ACS Appl. Mater. Interfaces, 2012, 4, 460 CAS.
- H. Kong and J. Jang, Chem. Commun., 2006, 3010 RSC.
- J. Alegret, T. Rindzevicius, T. Pakizeh, Y. Alaverdyan, L. Gunnarsson and M. Käll, J. Phys. Chem. C, 2008, 112, 14313 CAS.
- L. Gunnarsson, T. Rindzevicius, J. Prikulis, B. Kasemo and M. Käll, J. Phys. Chem. B, 2005, 109, 1079 CrossRef CAS PubMed.
- H. Kong, J. Song and J. Jang, Environ. Sci. Technol., 2010, 44, 5672 CrossRef CAS PubMed.
- J. Jiang and L. Zhang, Chem.–Eur. J., 2011, 17, 3710 CrossRef CAS PubMed.
- Y. B. Tian and J. Zhang, Catal. Surv. Asia, 2012, 16, 210 CrossRef PubMed.
- P. Wang, B. Huang, Y. Dai and M.-H. Whangbo, Phys. Chem. Chem. Phys., 2012, 14, 9813 RSC.
- H. Wang, D. W. Brandl, P. Nordlander and N. J. Halas, Acc. Chem. Res., 2006, 40, 53 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra45341c |
| This journal is © The Royal Society of Chemistry 2014 |