Iron catalyzed efficient synthesis of 2-arylbenzothiazoles from benzothiazole and olefins using environmentally benign molecular oxygen as oxidant†
Ashok B. Khemnar and
Bhalchandra M. Bhanage*
Department of Chemistry, Institute of Chemical Technology, Matunga, Mumbai-400019, India. E-mail: bm.bhanage@gmail.com; bm.bhanage@ictmumbai.edu.in; Fax: +91-22-33611020; Tel: +91-22-33612601
First published on 20th January 2014
Abstract
A novel protocol for iron catalyzed arylation of benzothiazole with olefins has been developed using molecular oxygen as a greener oxidant. The reaction worked smoothly using inexpensive and easily available iron as a catalyst for the synthesis of 2-arylbenzothiazole derivatives in good to excellent yields.
Introduction
Carbon–carbon bond formation reactions using transition metal catalysts have enormous importance for the synthesis of organic compounds. The benzothiazole derivatives are of much interest as antitumor, antiviral, and antimicrobial agents.1 In addition, drugs such as zopolrestat2 and riluzole3 also contain benzothiazole derivatives which are used for the treatment of diabetes. Furthermore, they are also used as an important building block in pharmaceuticals, agrochemicals and natural products.4 Hence, the development of simple and efficient methodology for the synthesis of benzothiazole derivatives has attracted much attention in the past decades. The conventional methods for the synthesis of benzothiazole derivatives typically involve the condensation of 2-aminothiophenol or by cross-coupling of benzothiazole with different moieties such as aldehydes,5 ketones,6 nitriles,7 esters,8 halides,9 amines,10 and sodium arylsulfinates.11 Subsequently, intramolecular cyclization of various moieties for arylation of benzothiazole were also developed.12 Whereas metal free protocol for arylation of benzothiazole with aldehyde or acid using potassium persulfate oxidant under the nitrogen atmosphere was also reported.13Recently, the synthesis of heteroaryl compounds using an iron catalyst has attracted much interest in the transition metal-catalyzed cross-coupling reactions. Liu et al. have reported the arylation14 and acylation15 of benzothiazole with aldehydes and ketones respectively using iron catalyst under oxygen atmosphere. Most recently, Deb et al. have synthesized 2-arylbenzothiazoles by cross coupling of benzothiazole with boronic acids using iron catalyst and potassium persulfate as an oxidant.16 Similarly, Song and co-workers reported the copper catalyzed synthesis of 2-arylbenzothiazoles.17 However, most of these protocols have limitations such as multistep synthesis, stoichiometric amount of inorganic oxidants, readily oxidizable 2-aminothiophenols, and need of inert atmosphere.
Hence, to develop economical and sustainable protocol for the arylation of heteroaryl compounds that operates under environmentally friendly condition is of great interest. In continuation of our research in the development of efficient catalytic system for arylation of heterocyclic moieties.9a Herein, we report an efficient and homogeneous methodology for the synthesis of 2-arylbenzothiazole using inexpensive and easily available iron catalyst under environmentally benign oxygen as oxidant (Scheme 1).
Results and discussion
Initially, to optimise the reaction conditions benzothiazole (1a) and styrene (2a) were chosen as a model substrate for the iron catalyzed arylation reaction. A series of experiments were carried out to study the effect of various reaction parameters such as catalysts, solvents, oxidants, temperature and time (Table 1). Firstly, we screened various iron catalysts under oxygen atmosphere as an oxidant for the model reaction (Table 1, entries 1–6). It was observed that among the various iron catalysts, ferric nitrate gave the good yield of the arylation product along with minor acylation product and hence was used for further studies (Table 1, entry 6). Encouraged by this result, we studied the effect of other oxidants such as TBHP (5–6 in decane), K2S2O8 and air (Table 1, entries 7–9), among the screened oxidants molecular oxygen furnished good yield of the desired product (Table 1, entry 6). As the correct combination of catalyst with ligands was crucial for such reactions, we performed reaction by using ferric nitrate with ligands like DPPM and P(t-Bu)3·HBF4 (Table 1, entries 10–11). It was found that P(t-Bu)3·HBF4 provided the excellent result (Table 1, entry 11). Furthermore, we studied the effect of catalyst as well as ligand concentration and it was found that 5 mol% catalyst and 25 mol% ligand furnished the excellent yield (Table 1, entry 12). Subsequently, we studied the effect of various solvents for this transformation (Table 1, entries 13–16). It was observed that the combination of DMSO and water was essential for present reaction (Table 1, entry 12). In addition, the effect of temperature and time were also investigated (Table 1, entries 17–18). It was found that 120 °C was the optimum temperature required for the arylation of benzothiazole (Table 1, entry 12). Whereas, yield of the product decreases with decrease in reaction time. Therefore, 24 h was the optimum time required for the completion of the reaction. Furthermore, we also examined the mole ratio of 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a and it was observed that 1 mmol of benzothiazole with 2.5 mmol of styrene i.e. 1
2a and it was observed that 1 mmol of benzothiazole with 2.5 mmol of styrene i.e. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 mole ratio furnished the highest yield of the desired arylation product (Table 1, entry 12).
2.5 mole ratio furnished the highest yield of the desired arylation product (Table 1, entry 12).
| Entry | Catalyst (mol%) | Ligand (mol%) | Solvent (ml) | Temp. (°C) | Yieldb (%) | |
|---|---|---|---|---|---|---|
| 3a | 4a | |||||
a Reaction conditions: 1a (1 mmol), 2a (2.5 mmol), catalyst (5–20 mol %), ligand (25 mol%), solvent (1.6 ml, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 120 °C, 24 h, under oxygen.b GC yield.c TBHP (5–6 M in decane).d K2S2O8.e Air atmosphere. 1), 120 °C, 24 h, under oxygen.b GC yield.c TBHP (5–6 M in decane).d K2S2O8.e Air atmosphere. |
||||||
| 1 | FeSO4·7H2O (20) | — | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
120 | 37 | 20 |
| 2 | Fe2(SO4)3·H2O (20) | — | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
120 | 17 | 05 |
| 3 | FeCl3 (20) | — | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
120 | 25 | 07 |
| 4 | Fe2O3 (20) | — | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
120 | 05 | 02 |
| 5 | Fe(OAc)2 (20) | — | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
120 | 07 | 02 |
| 6 | Fe(NO3)3·9H2O (20) | — | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
120 | 54 | 12 |
| 7c | Fe(NO3)3·9H2O (20) | — | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
120 | 22 | 09 |
| 8d | Fe(NO3)3·9H2O (20) | — | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
120 | 13 | 06 |
| 9e | Fe(NO3)3·9H2O (20) | — | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
120 | 03 | 00 |
| 10 | Fe(NO3)3·9H2O (20) | DPPM | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
120 | 43 | 07 |
| 11 | Fe(NO3)3·9H2O (20) | P(t-Bu)3·HBF4 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
120 | 74 | 04 |
| 12 | Fe(NO3)3·9H2O (5) | P(t-Bu)3·HBF4 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
120 | 88 | 05 |
| 13 | Fe(NO3)3·9H2O (5) | P(t-Bu)3·HBF4 | Diglyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
120 | 10 | 03 |
| 14 | Fe(NO3)3·9H2O (5) | P(t-Bu)3·HBF4 | DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
120 | 02 | 00 |
| 15 | Fe(NO3)3·9H2O (5) | P(t-Bu)3·HBF4 | DMSO | 120 | 04 | 00 |
| 16 | Fe(NO3)3·9H2O (5) | P(t-Bu)3·HBF4 | H2O | 120 | 15 | 06 |
| 17 | Fe(NO3)3·9H2O (5) | P(t-Bu)3·HBF4 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
130 | 89 | 08 |
| 18 | Fe(NO3)3·9H2O (5) | P(t-Bu)3·HBF4 | DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
110 | 74 | 05 |
Hence, the optimized reaction parameters for the arylation of benzothiazole are: benzothiazole (1a, 1 mmol), styrene (2a, 2.5 mmol), 5 mol% catalyst, P(t-Bu)3·HBF4 ligand (25 mol%), DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (3
H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
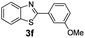
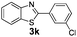
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.6 ml) solvent under the oxygen atmosphere at 120 °C for 24 h. With these optimized reaction parameters, the scope of developed protocol was further extended for the wide range of substrates. Various aromatic olefins bearing electron donating and withdrawing substituents on the phenyl ring were well tolerated under the present reaction condition and afforded the corresponding arylated products 3a–3n in good yield (Table 2). 3-Methyl styrene and 4-tert-butyl styrene were also provided the excellent yield of 3b and 3c products respectively (Table 2, entries 2–3). The reaction of benzothiazole with olefins having electron donating group provided the arylated products in good yields (Table 2, entries 4–6). Subsequently, we studied the impact of electronic and structural variations of substituents on the phenyl ring of olefins. The ortho-substituted olefins were well tolerated as compared to para-substituted olefins which indicate that there is no effect of steric hindrance (Table 2, entries 4–10). The meta-substituted olefin also furnished good yield (Table 2, entries 2, 6 and 11–12). The phenyl ring of olefin with halogen substituents like bromo, chloro were compatible under this procedure, and the desired arylated products were isolated in good yields (Table 2, entries 7–12). In addition, electron withdrawing group on the phenyl ring of olefin afforded the moderate yield (Table 2, entry 13). Furthermore, heteroaromatic olefin also provided the arylation product in moderate yield (Table 2, entries 14–15). However, aliphatic olefins were unreactive under the optimized reaction conditions. When optimized reaction condition applied for the reaction of 4,5-dimethylthiazole with styrene, arylated product was not observed (Table 2, entry 16). The reactions of benzoxazole and N-methyl benzimidazole were failed to provide the desired arylated product under present catalytic conditions.
1, 1.6 ml) solvent under the oxygen atmosphere at 120 °C for 24 h. With these optimized reaction parameters, the scope of developed protocol was further extended for the wide range of substrates. Various aromatic olefins bearing electron donating and withdrawing substituents on the phenyl ring were well tolerated under the present reaction condition and afforded the corresponding arylated products 3a–3n in good yield (Table 2). 3-Methyl styrene and 4-tert-butyl styrene were also provided the excellent yield of 3b and 3c products respectively (Table 2, entries 2–3). The reaction of benzothiazole with olefins having electron donating group provided the arylated products in good yields (Table 2, entries 4–6). Subsequently, we studied the impact of electronic and structural variations of substituents on the phenyl ring of olefins. The ortho-substituted olefins were well tolerated as compared to para-substituted olefins which indicate that there is no effect of steric hindrance (Table 2, entries 4–10). The meta-substituted olefin also furnished good yield (Table 2, entries 2, 6 and 11–12). The phenyl ring of olefin with halogen substituents like bromo, chloro were compatible under this procedure, and the desired arylated products were isolated in good yields (Table 2, entries 7–12). In addition, electron withdrawing group on the phenyl ring of olefin afforded the moderate yield (Table 2, entry 13). Furthermore, heteroaromatic olefin also provided the arylation product in moderate yield (Table 2, entries 14–15). However, aliphatic olefins were unreactive under the optimized reaction conditions. When optimized reaction condition applied for the reaction of 4,5-dimethylthiazole with styrene, arylated product was not observed (Table 2, entry 16). The reactions of benzoxazole and N-methyl benzimidazole were failed to provide the desired arylated product under present catalytic conditions.
| Entry | Thiazole | Olefin | Product | Yieldb (%) |
|---|---|---|---|---|
a Reaction conditions: 1 (1 mmol), 2 (2.5 mmol), Fe(NO3)3·9H2O (5 mol %), P(t-Bu)3·HBF4 (25 mol%), DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1.6 ml, 3 H2O (1.6 ml, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 120 °C, 24 h, under oxygen.b Isolated yield. 1), 120 °C, 24 h, under oxygen.b Isolated yield. |
||||
| 1 |  |
 |
 |
81 |
| 2 | 1a | 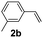 |
 |
80 |
| 3 | 1a |  |
 |
84 |
| 4 | 1a |  |
 |
72 |
| 5 | 1a |  |
 |
60 |
| 6 | 1a | 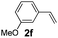 |
 |
75 |
| 7 | 1a |  |
 |
73 |
| 8 | 1a | 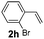 |
 |
66 |
| 9 | 1a |  |
 |
69 |
| 10 | 1a |  |
 |
60 |
| 11 | 1a |  |
 |
77 |
| 12 | 1a | 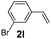 |
 |
68 |
| 13 | 1a |  |
 |
57 |
| 14 | 1a |  |
 |
42 |
| 15 | 1a | 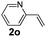 |
 |
44 |
| 16 |  |
 |
 |
00 |
To explore the reaction mechanism some control experiments have been carried out. Recently, Friedrich and Hong reported the conversion of styrene into benzaldehyde18 or benzoic acid19 respectively. Based on the above reports, we have carried out the reaction of benzaldehyde and benzoic acid with the benzothiazole under the optimized reaction condition. Only benzaldehyde provided the 92% 3a arylation product (Scheme 2); whereas the benzoic acid does not work (ESI, Scheme S1†). It was found that styrene was converted into the benzaldehyde using ferric nitrate catalyst in the oxygen atmosphere (Scheme 2). Additionally, benzothiazole converted into the 3% of 2-aminothiophenol and 10% dimer of 2-aminothiophenol (ESI, Scheme S1†). The reaction of 2-aminothiophenol and styrene were carried out to get an idea whether the reaction was going through the ring opening pathway or not and it was observed that 2-arylbenzothiazole with 2-benzoylbenzothiazole was obtained in 35% and 08% yield respectively (ESI, Scheme S1†). These results indicate that the reaction may be proceeding through ring opening pathway. When the reaction of benzothiazole and styrene were carried out in the presence of radical scavenger TEMPO the formation of arylation (3a) product was not observed (ESI, Scheme S1†).
Based on our experimental observation, a plausible reaction mechanism for the arylation of benzothiazole was shown in Scheme 3. Firstly the benzothiazole was transformed into the 2-aminothiophenol (A) through ring opening reaction in the presence of iron catalyst. In the meanwhile, styrene is oxidized to aldehyde (B). In the subsequent step, 2-aminothiophenol condensed with benzaldehyde giving the imine (C) which undergoes intramolecular cyclization provided the intermediate (D). Finally, oxidative dehydrogenation of (D) afforded the arylation product 3a.
Conclusion
In conclusion, we have developed a novel, simple, efficient and useful protocol for the arylation of benzothiazole with various olefins using iron as a catalyst and oxygen as oxidant. In addition the developed methodology has significant advantages as compared to the earlier methods such as (i) first time arylation of benzothiazole by using olefin was successfully achieved, (ii) use of less toxic and inexpensive iron catalyst compared with other transition metals, (iii) use of oxygen as an oxidant, (iv) wider substrate applicability. Thus, the developed catalytic system constitutes a highly efficient, economically attractive and environmentally favourable process for the synthesis of 2-arylbenzothiazoles. Further application of this catalytic system and the detailed mechanistic study is under progress.Experimental
General procedure for the synthesis of 2-arylbenzothiazole
To an oven dried 15 ml glass vial with a magnetic bar was charged with benzothiazole (1a, 1 mmol), styrene (2a, 2.5 mmol), ferric nitrate (5 mol %), P(t-Bu)3·HBF4 (25 mol%), and solvent (DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, 3
H2O, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The vial was then flush with oxygen and sealed with a cap. The reaction mixture was stirred at 120 °C for 24 h monitored by TLC and GC. After completion, cool the reaction mixture to room temperature. Extract the product with ethyl acetate (3 × 15 ml), dried the organic layer over Na2SO4 and evaporated to afford the crude product. The product was purified by column chromatography (silica gel, 100–200 mesh; petroleum ether/ethyl acetate) to afford the pure product. The product was confirmed by GC-MS, 1H and 13C NMR spectroscopic analysis.
1). The vial was then flush with oxygen and sealed with a cap. The reaction mixture was stirred at 120 °C for 24 h monitored by TLC and GC. After completion, cool the reaction mixture to room temperature. Extract the product with ethyl acetate (3 × 15 ml), dried the organic layer over Na2SO4 and evaporated to afford the crude product. The product was purified by column chromatography (silica gel, 100–200 mesh; petroleum ether/ethyl acetate) to afford the pure product. The product was confirmed by GC-MS, 1H and 13C NMR spectroscopic analysis.
Acknowledgements
The author A. B. Khemnar is greatly thankful to Council of Scientific and Industrial Research (CSIR) India for providing senior research fellowship (SRF).Notes and references
- (a) L. W. Wattenberg, M. A. Page and J. L. Leong, Cancer Res., 1968, 28, 2539 CAS; (b) R. B. Trigg, J. V. Warrington and P. J. Palmer, J. Med. Chem., 1971, 14, 248 CrossRef; (c) S. E. O'Brien, H. L. Browne, T. D. Bradshaw, A. D. Westwell, M. F. G. Stevens and C. A. Laughton, Org. Biomol. Chem., 2003, 1, 493 RSC; (d) T. D. Bradshaw and A. D. Westwell, Curr. Med. Chem., 2004, 11, 1009 CrossRef CAS; (e) M. Chakraborty, K. J. Jin, S. C. Brewer III, H.-L. Peng, M. S. Platz and M. Novak, Org. Lett., 2009, 11, 4862 CrossRef CAS PubMed; (f) P. P. Nandekar, K. M. Tumbi, N. Bansal, V. P. Rathod, L. B. Labhsetwar, N. Soumya, S. Singh and A. T. Sangamwar, Med. Chem. Res., 2013, 22, 3728 CrossRef CAS.
- T. A. Beyer, P. J. Scott, C. E. Aldinger, M. F. Dee, T. W. Siegel, W. J. Zembrowski and B. L. Mylari, J. Med. Chem., 1992, 35, 457 CrossRef.
- J.-H. Song, C.-S. Huang, K. Nagata, J. Z. Yeh and T. Narahashi, J. Pharmacol. Exp. Ther., 1997, 282, 707 CAS.
- (a) C. J. Paget, K. Kisner, R. L. Stone and D. C. DeLong, J. Med. Chem., 1969, 12, 1016 CrossRef CAS; (b) V. G. Shirke, A. S. Bobad, R. P. Bhamaria, B. G. Khadse and S. R. Sengupta, Indian Drugs, 1990, 27, 350 CAS; (c) T. Kondo and T.-A. Mitsudo, Chem. Rev., 2000, 100, 3205 CrossRef CAS PubMed; (d) R. H. Tale, Org. Lett., 2002, 4, 10 CrossRef PubMed; (e) I. P. Beletskaya and V. P. Ananikov, Chem. Rev., 2011, 111, 1596 CrossRef CAS PubMed; (f) Y. Liu and J.-P. Wan, Org. Biomol. Chem., 2011, 9, 6873 RSC.
- K. Bahrami, M. M. Khodaei and F. Naali, J. Org. Chem., 2008, 73, 6835 CrossRef CAS PubMed.
- Y. Liao, H. Qi, S. Chen, P. Jiang, W. Zhou and G.-J. Deng, Org. Lett., 2012, 14, 6004 CrossRef CAS PubMed.
- Y. Sun, H. Jiang, W. Wu, W. Zeng and X. Wu, Org. Lett., 2013, 15, 1598 CrossRef CAS PubMed.
- S. Rostamizadeh, M. Nojavan and F. Heshmatpoor, Heterocycl. Commun., 2007, 13, 305 CrossRef CAS.
- (a) N. S. Nandurkar, M. J. Bhanushali, M. D. Bhor and B. M. Bhanage, Tetrahedron Lett., 2008, 49, 1045 CrossRef CAS PubMed; (b) J. Canivet, J. Yamaguchi, I. Ban and K. Itami, Org. Lett., 2009, 11, 1733 CrossRef CAS PubMed; (c) J. Huang, J. Chan, Y. Chen, C. J. Borths, K. D. Baucom, R. D. Larsen and M. M. Faul, J. Am. Chem. Soc., 2010, 132, 3674 CrossRef CAS PubMed; (d) T. Yamamoto, K. Muto, M. Komiyama, J. Canivet, J. Yamaguchi and K. Itami, Chem. – Eur. J., 2011, 17, 10113 CrossRef CAS PubMed; (e) W. Zhang, Q. Zeng, X. Zhang, Y. Tian, Y. Yue, Y. Guo and Z. Wang, J. Org. Chem., 2011, 76, 4741 CrossRef CAS PubMed; (f) J. Yamaguchi, K. Muto and K. Itami, Eur. J. Org. Chem., 2013, 19 CrossRef CAS.
- (a) F. Su, S. C. Mathew, L. Mohlmann, M. Antonietti, X. Wang and S. Blechert, Angew. Chem., Int. Ed., 2011, 50, 657 CrossRef CAS PubMed; (b) T. B. Nguyen, L. Ermolenko, W. A. Dean and A. Al-Mourabit, Org. Lett., 2012, 14, 5948 CrossRef CAS PubMed; (c) Z. Yang, A. Wang, X. Chen, Q. Gui, J. Liu, Z. Tan, H. Wang and J.-C. Shi, Synlett, 2013, 24, 1549 CrossRef CAS PubMed; (d) T. Xiao, S. Xiong, Y. Xie and X. D. L. Zhou, RSC Adv., 2013, 3, 15592 RSC.
- M. Wang, D. Li, W. Zhou and L. Wang, Tetrahedron, 2012, 68, 1926 CrossRef CAS PubMed.
- (a) L. L. Joyce, G. Evindar and R. A. Batey, Chem. Commun., 2004, 446 RSC; (b) D. Ma, S. Xie, P. Xue, X. Zhang, J. Dong and Y. Jiang, Angew. Chem., Int. Ed., 2009, 48, 4222 CrossRef CAS PubMed; (c) H. Wang, L. Wang, J. Shang, X. Li, H. Wang, J. Gui and A. Lei, Chem. Commun., 2012, 48, 76 RSC; (d) Y. Cheng, J. Yang, Y. Qu and P. Li, Org. Lett., 2012, 14, 98 CrossRef CAS PubMed.
- Z. Yang, X. Chen, S. Wang, J. Liu, K. Xie, A. Wang and Z. Tan, J. Org. Chem., 2012, 77, 7086 CrossRef CAS PubMed.
- S. Liu, R. Chen, X. Guo, H. Yang, G. Deng and C.-J. Li, Green Chem., 2012, 14, 1577 RSC.
- S. Liu, R. Chen, H. Chen and G.-J. Deng, Tetrahedron Lett., 2013, 54, 3838 CrossRef CAS PubMed.
- A. Deb, S. Manna, A. Maji, U. Dutta and D. Maiti, Eur. J. Org. Chem., 2013, 5251 CrossRef CAS.
- Q. Song, Q. Feng and M. Zhou, Org. Lett., 2013, 15, 5990–5993 CrossRef CAS PubMed.
- J. Valand, H. Parekh and H. B. Friedrich, Catal. Commun., 2013, 40, 149–153 CrossRef CAS PubMed.
- T. M. Shaikha and F.-E. Hong, Adv. Synth. Catal., 2011, 353, 1491–1496 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra46955g |
| This journal is © The Royal Society of Chemistry 2014 |





