ZnNi alloy nanoparticles grown on reduced graphene oxide nanosheets and their magnetic and catalytic properties†
Jinglei
Yang
a,
Xiaoping
Shen
*a,
Guoxing
Zhu
a,
Zhenyuan
Ji
b and
Hu
Zhou
b
aSchool of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, P. R. China. E-mail: xiaopingshen@163.com; Fax: +86 511-88791800; Tel: +86 511-88791800
bSchool of Materials Science and Engineering, Jiangsu University, Zhenjiang 212013, P. R. China
First published on 20th November 2013
Abstract
Reduced graphene oxide (RGO)–ZnNi alloys nanocomposites were synthesized by in situ growth of ZnNi alloy nanocrystals on graphene oxide (GO) accompanied with a chemical co-reduction process. The size and morphology of ZnNi alloy nanoparticles on RGO sheets can be tuned by simply changing initial concentrations of metal ions and molar ratio of Zn2+ to Ni2+ in the reaction system. The as-synthesized products were characterized by powder X-ray diffraction, energy dispersive X-ray spectroscopy, Raman spectroscopy and transmission electron microscopy. Magnetic measurements reveal that the nanocomposites have ferromagnetic characteristics and show composition dependent magnetic properties. Moreover, the RGO–ZnNi nanocomposites show remarkably enhanced catalytic activity and recycling stabilities toward the reduction of 4-nitrophenol (4-NP), and can be easily re-collected from the reaction system by a magnet. It is believed that the obtained magnetic RGO–ZnNi nanocomposites with excellent catalytic properties provide a new opportunity for the application of graphene-based materials.
1. Introduction
Metal nanomaterials, as one kind of the most important materials, have received wide recognition due to their unique properties and wide applications in various fields.1 Especially, bimetallic nanocrystals composed of two different metal elements possess interesting size- and composition-dependent electrical, chemical and optical properties.2–5 Recently, more and more interest is being endowed into the field of alloy catalysis owing to the enhanced performance compared to their individual components. For example, Y. Huang et al. reported that Pt3Ni nanocrystals can serve as highly efficient catalysts in oxygen reduction reaction with much better performance and durability than those of the commercial Pt black and commercial Pt/C catalysts.6 Since the reduction of aromatic nitro compounds to amines is a very vital process in synthetic organic chemistry and in industrial fabrication of many industrially important products, the reduction of 4-NP into 4-aminophenol (4-AP) by NaBH4 has been widely employed as a model reaction to quantitatively evaluate the catalytic activity of a variety of metal or alloy catalysts.7–10 For instance, K. Mandal et al. reported that NiCo alloy nanochains show excellent catalytic activity in the borohydride reduction of 4-NP.10 These studies provide an important hint that bimetal or alloy nanoparticles would have excellent catalytic performance on the reduction of 4-NP. As for alloys, we are interested in ZnNi alloys, which have been widely used in many fields because of their excellent corrosion resistance.11-13 Recently, ZnNi alloys are found to be a new type of catalytic materials possessing high catalytic activity.14,15 The unsupported ZnNi has been used as methanol steam reforming catalysts by Friedrich et al.14 ZnNi catalysts supported on TiO2, TiO2–SiO2, TiO2/SBA-15 and SBA-15 substrates have been prepared by Loricera et al. and they show superior catalytic activities in the hydrodeoxygenation of phenol and the hydrogenation of synthetic diesel.16Graphene, an atom-thick two-dimensional (2D) carbon material, is emerging as one of the most appealing catalytic support materials due to its unique structure and excellent properties such as superior electrical conductivity, excellent mechanical flexibility, high thermal and chemical stability,17 extremely high surface area (theoretical value of 2630 m2 g−1).18 The integration of graphene with other materials including metal,19 semiconductor,20 ceramics,21 polymer22 and other carbon materials23 is becoming a promising approach to harness the favorable properties of graphene for practical applications. It has been demonstrated that graphene-supported FePt,24 FeAu,25 PtAu,26 AgAu,27 and FeNi28 exhibit unusually high catalytic performance, which makes graphene an ideal substitute for other carbon materials as catalyst support. However, to our knowledge, the integration of nano-sized ZnNi with graphene has not been reported so far. Herein, we develop an easy wet chemical route to grow ZnNi nanocrystals on reduced graphene oxide (RGO) nanosheets for the first time. The morphology and size of ZnNi alloy on RGO sheets can be adjusted by simply changing initial metal ions concentrations and molar ratio of Zn2+ to Ni2+. The graphene-based RGO–ZnNi nanocomposites show ferromagnetic property and excellent catalytic performance toward the reduction of 4-NP.
2. Experimental
2.1. Materials
Natural flake graphite with a particle size of 150 μm (99.9% purity) was purchased from Qingdao Guyu Graphite Co., Ltd. All of the other chemical reagents used in our experiments were of analytical grade, purchased from Sinopharm Chemical Reagent Co., Ltd, and used without further purification. Graphite oxide was prepared from the natural flake graphite according to a modified Hummers method.29,302.2. Preparation of ZnNi alloys on RGO
The typical procedure for the preparation of the alloy of ZnNi5 nanoparticles attached on RGO nanosheets is as follows: 50 mg of GO was sonicated in 140 mL of ethylene glycol (EG) to form a uniform GO dispersion. Subsequently, 0.84 mmol of Ni(NO3)2·6H2O and 0.17 mmol of Zn(NO3)2·6H2O (with initial molar ratio of Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 1
Ni = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) were dispersed in 10 mL of EG, and then gradually added into the GO dispersion. The resulting mixture was magnetically stirred at room temperature for 1 h, and heated to 110 °C under N2 atmosphere. Then, 25 mL of hydrazine hydrate dissolved with 1 g of NaOH was slowly added in, and the resulted mixture was refluxed at 110 °C for 45 min. After the reaction system was naturally cooled to room temperature, the black precipitates were collected by centrifugation, washed with distilled water and absolute ethanol several times, and dried in a vacuum oven at 45 °C for 24 h. For comparison, ZnNi alloy nanoparticles with different sizes and compositions were grown on RGO nanosheets by adjusting the initial concentrations and molar ratios of Zn2+ and Ni2+ ions. In addition, RGO–Ni, bare ZnNi5, and pure RGO were also prepared in the same experimental conditions in the absence of Zn(NO3)2·6H2O, GO, and both Zn(NO3)2·6H2O and Ni(NO3)2·6H2O, respectively. Detailed parameters for these synthetic experiments are shown in Table 1.
5) were dispersed in 10 mL of EG, and then gradually added into the GO dispersion. The resulting mixture was magnetically stirred at room temperature for 1 h, and heated to 110 °C under N2 atmosphere. Then, 25 mL of hydrazine hydrate dissolved with 1 g of NaOH was slowly added in, and the resulted mixture was refluxed at 110 °C for 45 min. After the reaction system was naturally cooled to room temperature, the black precipitates were collected by centrifugation, washed with distilled water and absolute ethanol several times, and dried in a vacuum oven at 45 °C for 24 h. For comparison, ZnNi alloy nanoparticles with different sizes and compositions were grown on RGO nanosheets by adjusting the initial concentrations and molar ratios of Zn2+ and Ni2+ ions. In addition, RGO–Ni, bare ZnNi5, and pure RGO were also prepared in the same experimental conditions in the absence of Zn(NO3)2·6H2O, GO, and both Zn(NO3)2·6H2O and Ni(NO3)2·6H2O, respectively. Detailed parameters for these synthetic experiments are shown in Table 1.
| Samples | Initial feeding amount of metal ions (mmol) | Zn2+/Ni2+ molar ratio | ZnNi contenta wt% |
|---|---|---|---|
| a The contents of ZnNi alloys in the samples were determined by ICP-OES. | |||
| RGO–ZnNi5-1 | n(Zn2+) + n(Ni2+) = 1.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
70.87 |
| RGO–ZnNi5-2 | n(Zn2+) + n(Ni2+) = 1.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
55.73 |
| RGO–ZnNi5-3 | n(Zn2+) + n(Ni2+) = 0.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
50.03 |
| RGO–ZnNi4 | n(Zn2+) + n(Ni2+) = 1.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
59.76 |
| RGO–ZnNi3 | n(Zn2+) + n(Ni2+) = 1.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
62.74 |
| RGO–ZnNi2 | n(Zn2+) + n(Ni2+) = 1.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
66.17 |
| RGO–Ni | n(Ni2+) = 1.0 | — | 57.64 |
| ZnNi5 | n(Zn2+) + n(Ni2+) = 1.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
100 |
2.3. Instrumentation and measurements
The phase structures of the as-synthesized products were characterized using X-ray diffraction (XRD, Bruker D8 ADVANCE) with Cu-Kα radiation (λ = 1.5406 Å) at a scanning rate of 4° min−1. The morphology and the size of the products were examined by field emission scanning electron microscopy (FESEM, JSM-7001F) and transmission electron microscopy (TEM, JEM-2100). Samples for TEM were prepared by dropping the products on a carbon-coated copper grid after ultrasonic dispersion in absolute ethanol and allowed to dry in air before analysis. The compositions of the products were determined by energy-dispersive X-ray spectrometry (EDS) and inductively coupled plasma optical emission spectrometry (ICP-OES, Vista-MPX). EDS was recorded with an energy dispersive spectrometer attached to the scanning electron microscope (JSM-6480). Fourier transform infrared (FT-IR) spectra were recorded on a Nicolet Nexus 470 spectrometer with KBr pellets. Raman scattering was performed on a JY-HR800 Raman spectrometer using a 453 nm laser source. The magnetic measurements were performed using a vibrating sample magnetometer (VSM, Nanjing University HH-15) at room temperature (300 K). Ultraviolet-visible (UV-vis) spectroscopy measurements were performed on a UV-2450 ultraviolet-visible spectrophotometer.2.4. Catalytic reduction of 4-NP
The reduction reaction of 4-NP by NaBH4 was used as a model system to quantitatively evaluate the catalytic activity of the as-synthesized nanocomposites. In a typical reaction, 5 mg of the as-synthesized samples and 2 mL of freshly prepared NaBH4 solution (1.5 mM) were added to 100 mL of DI water. After stirred for 15 min at 25 °C, 1 mL of 4-NP solution (5 mM) was injected into the mixture to start the reaction. During the reaction process, 2.0 mL of reaction solution was withdrawn at a given time interval, which was immediately recorded in the UV-vis spectrophotometer in a scanning range of 250–500 nm at 25 °C. For successive recycling of the catalysts, the catalysts were magnetically separated from the solution, and then were added into another freshly prepared mixed solution of 2 mL of NaBH4 solution and 1.0 mL of 4-NP solution to start the next round of reaction.3. Results and discussion
3.1. Characterization of RGO–ZnNi
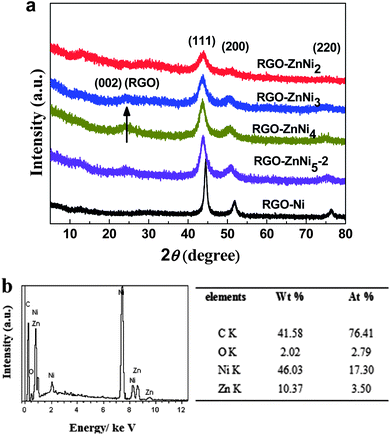
In this study, RGO–ZnNi nanocomposites were prepared through a simple one-pot co-reduction method, in which Zn2+, Ni2+ and GO were reduced by hydrazine hydrate simultaneously. As we all know, GO sheets are highly negatively charged when dispersed in EG because of the ionization of the carboxylic acid and phenolic hydroxyl groups that exist on their edge and surface.31 When positively charged Zn2+ and Ni2+ ions and negatively charged GO sheets were introduced together, the electrostatic interactions between these components provide a strong driving force for the effective adsorption of Zn2+ and Ni2+ onto GO sheets. With the addition of hydrazine hydrate, Zn2+ and Ni2+ ions were in situ reduced into ZnNi alloy on GO surface and GO was reduced into RGO simultaneously, as a result, RGO–ZnNi nanocomposites were formed.The XRD patterns of the as-prepared samples are shown in Fig 1a. The diffraction pattern of RGO–Ni exhibits three peaks at 2θ = 44.31°, 51.64° and 75.94°, corresponding to the (111), (200) and (220) crystal planes of face-centered cubic (fcc) Ni (JCPDS 01-1260). The XRD patterns of RGO–ZnNi nanocomposites are very similar to that of RGO–Ni, but with a slight shift of peak positions towards lower angles. This is reasonable because the atomic radius of Zn is slightly bigger than that of Ni, and the substitution of Zn for Ni in the Ni-based fcc lattice results in the increase of the lattice constants and the spacings of crystal planes.32,33 The diffraction peak intensity decreases with the increasing of Zn content, indicating that a higher Zn content in the ZnNi alloys will lead to weaker crystallinity. The characteristic (001) peak at about 10° for graphite oxide disappears in all the diffraction patterns, suggesting that graphite oxide has been flaked in these composites. The characteristic (002) peak of RGO at around 24° is very weak for all the composites, suggesting that the GO is reduced to RGO and the restacking of RGO sheets has been prevented by the metal alloy nanoparticles on them.34 Meanwhile, no zinc and nickel oxides or hydroxides or other impurity phases are detected in these XRD patterns. EDS spectrum of the typical composite of RGO–ZnNi5-2 is shown in Fig 1b. The detectable elements in RGO–ZnNi5-2 includes zinc, nickel, carbon and oxygen, and the Zn/Ni molar ratio obtained from the spectrum is close to the initial Zn2+/Ni2+ molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The carbon element would come from the RGO nanosheets, while oxygen mainly from the residual oxygen-containing functional groups on RGO. The actual contents and chemical compositions of the ZnNi alloys in RGO–ZnNi nanocomposites were further determined by ICP-OES. It was found that the actual compositions of the ZnNi alloys in these composites are in agreement with the initial molar ratios of metal ions (Table 1).
5. The carbon element would come from the RGO nanosheets, while oxygen mainly from the residual oxygen-containing functional groups on RGO. The actual contents and chemical compositions of the ZnNi alloys in RGO–ZnNi nanocomposites were further determined by ICP-OES. It was found that the actual compositions of the ZnNi alloys in these composites are in agreement with the initial molar ratios of metal ions (Table 1).
 | ||
| Fig. 1 (a) XRD patterns of the as-prepared nanocomposites. (b) EDS spectrum of RGO–ZnNi5-2 nanocomposites. | ||
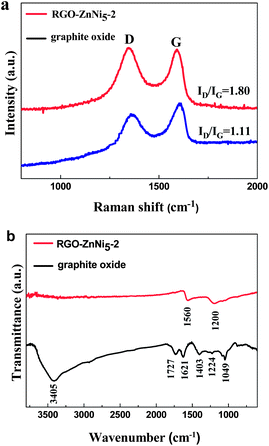
Raman spectroscopy has been widely employed to characterize carbon materials and to distinguish between different forms of ordered and disordered carbon as well as to extract physical parameters of relevance.35,36 Here, Raman spectroscopy was used to characterize the structural properties of graphite oxide and RGO in the RGO–ZnNi nanocomposites. As shown in Fig. 2a, the Raman spectrum of graphite oxide displays two prominent peaks at ca. 1350 and 1604 cm−1, corresponding to the well-documented D and G bands, respectively, while for the sample of RGO–ZnNi5-2, the G band moves to ca.1580 cm−1, close to the value of the pristine graphite. It is well known that the G band is usually assigned to the E2g mode observed for sp2 carbon domains, while the D band is associated with structural defects and disorders that can break the symmetry and selection rule.37 The intensity ratio of D to G (ID/IG) is inversely proportional to the average crystallite size of graphite materials.38 The ID/IG ratio varies from 1.11 for graphite oxide to 1.80 for RGO–ZnNi5-2 nanocomposites. The increased ID/IG ratio suggests the conjugated graphene network (sp2 carbon) is re-established after chemical reduction; however, the size of the re-established graphene network is usually smaller than the original graphite oxide, which will consequently lead to an increase of ID/IG.39 These results reveal that GO in RGO–ZnNi nanocomposites has been well deoxygenated and reduced.
Fig. 2b shows the FT-IR spectra of graphite oxide and RGO–ZnNi5-2 nanocomposites. For graphite oxide, the absorption bands at 3405 and 1403 cm−1 can be assigned to the stretching vibration and deformation vibration of O–H, respectively. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the COOH groups situated at the edges of graphene oxide sheets is found at 1727 cm−1.40 The bands at 1224 and 1049 cm−1 correspond to the stretching vibration peaks of C–O (epoxy) and C–O (alkoxy), respectively. The band at 1621 cm−1 derives from the vibrations of the adsorbed water molecules and also the contributions of the skeletal vibrations of unoxidized graphitic domains.41 For the RGO–ZnNi5-2 nanocomposites, most bands that related with the oxygen-containing functional groups vanish in the FT-IR spectrum except a weak peak at approximately 1200 cm−1 (ν(C–O)),42 revealing that the GO was effectively deoxygenated and reduced during the reduction process. The new absorption band at 1560 cm−1 can be assigned to the skeletal vibration of the graphene sheets.
O stretching vibration of the COOH groups situated at the edges of graphene oxide sheets is found at 1727 cm−1.40 The bands at 1224 and 1049 cm−1 correspond to the stretching vibration peaks of C–O (epoxy) and C–O (alkoxy), respectively. The band at 1621 cm−1 derives from the vibrations of the adsorbed water molecules and also the contributions of the skeletal vibrations of unoxidized graphitic domains.41 For the RGO–ZnNi5-2 nanocomposites, most bands that related with the oxygen-containing functional groups vanish in the FT-IR spectrum except a weak peak at approximately 1200 cm−1 (ν(C–O)),42 revealing that the GO was effectively deoxygenated and reduced during the reduction process. The new absorption band at 1560 cm−1 can be assigned to the skeletal vibration of the graphene sheets.
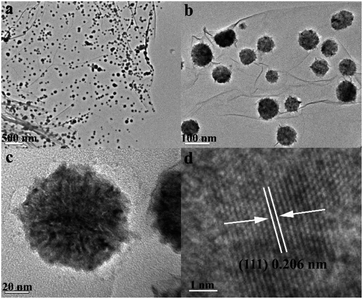
To investigate the morphology and microstructure of ZnNi alloys supported on RGO, TEM characterization was performed for several typical samples. Fig 3 shows the TEM images of RGO–ZnNi5-2 at different magnifications. RGO sheets can be clearly distinguished from the background and are nearly smooth with a few wrinkles, which is well consistent with the bare RGO synthesized in the absence of Zn(NO3)2·6H2O and Ni(NO3)2·6H2O (Fig. S1†), and the size of the bare RGO nanosheets can be seen to be as large as several micrometers (Fig. S1†). The ZnNi5 alloy nanoparticles are homogeneously anchored onto the surface of the RGO sheets and are well separated from each other although some of them tend to assemble into chain-like structure. As shown in Fig. 3b, the mean size of the ZnNi5 nanoparticles is 51.7 nm (Fig. 4d). The good distribution of ZnNi5 alloy nanoparticles on graphene sheets guarantees the efficient catalytic properties of RGO–ZnNi5 alloy nanomaterials. Fig. 3c shows a typical individual ZnNi5 nanoparticle. HRTEM image of the ZnNi5 nanoparticle is shown in Fig. 3d, which reveals the polycrystalline characteristic of the alloy nanoparticle. The lattice spacing of 0.206 nm for (111) plane of ZnNi5 is larger than that (0.203 nm) of pure Ni,28 which is consistent with the result of XRD (Fig. 1). For comparison, TEM image of the bare ZnNi5 prepared in the absence of GO is shown in Fig. 4g. The product consists of ZnNi5 microspheres with an average diameter of 618.1 nm (Fig. 4h), which is much bigger than the ZnNi5 nanoparticles in RGO–ZnNi5-2, exhibiting that GO nanosheets have a significant influence on the shape and size of the ZnNi alloy nanoparticles. This further confirms that GO nanosheets can play an important role in the nucleation and growth of inorganic nanocrystals.23,43
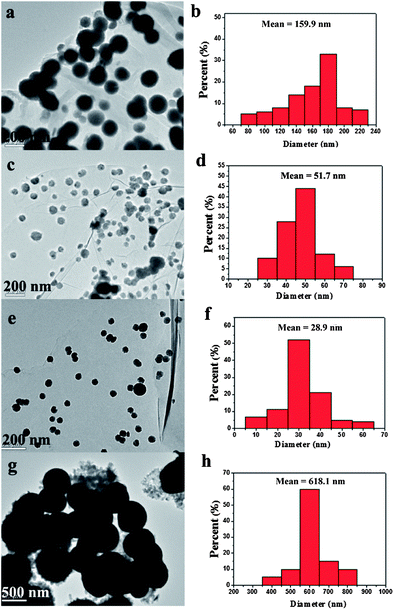 | ||
| Fig. 4 TEM images and the ZnNi particle size distribution of the (a and b) RGO–ZnNi5-1, (c and d) RGO–ZnNi5-2, (e and f) RGO–ZnNi5-3 and (g and h) the bare ZnNi5. | ||
In addition, it was found that the size and morphology of the ZnNi alloy nanocrystals on RGO nanosheets strongly depend on the molar ratio of Zn2+ to Ni2+ (keeping the total mole of metal ions constant: n (Zn2+) + n (Ni2+) = 1.0 mmol). As shown in Fig. 5, the size and morphology of the ZnNi nanoparticles show a significant change with the increasing content of zinc. The average diameters of the alloy nanoparticles for RGO–Ni, RGO–ZnNi4, RGO–ZnNi3 and RGO–ZnNi2 are 36.7 (Fig. 5c), 75.9 (Fig. 5f), 127.8 (Fig. 5i) and 132.7 nm (Fig. 5l), respectively. The diameter increases with the increasing of Zn/Ni atomic ratio. These nanoparticles are all made up of smaller nanocrystals. Meanwhile, with different Zn/Ni ratio, the morphologies of the ZnNi alloy nanoparticles are also slightly different. The pure Ni nanoparticles exhibit irregular shape, while the ZnNi alloy nanoparticles show regular spherical shape structure, the surface of which becomes smoother with the increasing of Zn content. This result is consistent with that observed in the NiCo alloy nanoparticles.43 With the increasing of Ni content, the ZnNi alloy nanoparticles will be affected more markedly by the magnetocrystalline anisotropy,33 which results in the coarse surface or even irregular shape of them.
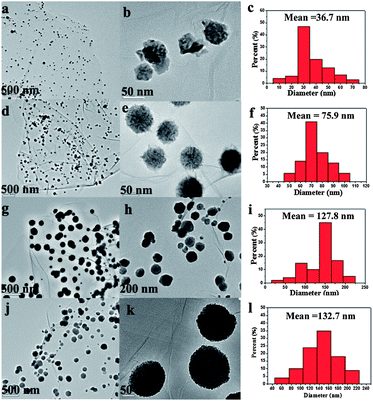 | ||
| Fig. 5 TEM images and ZnNi particle size distributions of (a–c) RGO–Ni, (d–f) RGO–ZnNi4, (g–i) RGO–ZnNi3 and (j–l) RGO–ZnNi2. | ||
Moreover, the size of the ZnNi nanoparticles on RGO sheets can also be affected by the amount of metal ions used. As shown in Table 1, the samples of RGO–ZnNi5-1, RGO–ZnNi5-2 and RGO–ZnNi5-3 were synthesized with the same Zn/Ni ion ratio but different amounts. The corresponding TEM images and the detailed particle size distribution of the ZnNi particle are shown in Fig. 4. It can be clearly seen that ZnNi nanoparticles are well loaded on RGO nanosheets in all these samples. The average sizes of ZnNi particles in RGO–ZnNi5-1, RGO–ZnNi5-2 and RGO–ZnNi5-3 are 159.9 (Fig. 4b), 51.7 (Fig. 4d) and 28.9 nm (Fig.4f), respectively, revealing that the less dosage of metal ions will lead to the smaller size of ZnNi5 nanoparticles, which is also consistent with our previous report.28 Also, the distribution density of ZnNi5 nanoparticles on RGO sheets shows an obvious decrease with the decreasing amount of metal ions.
3.2. Magnetic properties of RGO–ZnNi nanocomposites
Magnetic properties of the as-prepared nanocomposites were investigated at room temperature (300 K) using a vibrating sample magnetometer with an applied field −10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe ≤ H ≤ 10
000 Oe ≤ H ≤ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe. For quantitative comparison of our data, we normalized the measured magnetization data with respect to the ZnNi contents in the samples determined by ICP-OES. Thus all our magnetization data are given in the unit of emu per gram of ZnNi alloys. All of the samples exhibit typical ferromagnetic curves (Fig. 6), the values of saturation magnetization (Ms), remanence (Mr), remanence-to-saturation ratio (Mr/Ms)and coercivity (Hc) are listed in Table 2. As shown in Fig. 6a, RGO–ZnNi5-1, RGO–ZnNi5-2 and RGO–ZnNi5-3 show the similar Ms values. However, the Ms value of the bare ZnNi5 particles (Fig. 6a) is slightly bigger than those of the RGO–ZnNi5 nanocomposites, which could be attributed to the bigger particle size.31 Because of similar shape anisotropy, these samples show similar Hc values (70–80 Oe) except that RGO–ZnNi5-3 has a relatively higher Hc value. The bigger Hc value may originate from its smaller size, which may change the magnetization reversal mechanism and lead to a higher coercivity.44
000 Oe. For quantitative comparison of our data, we normalized the measured magnetization data with respect to the ZnNi contents in the samples determined by ICP-OES. Thus all our magnetization data are given in the unit of emu per gram of ZnNi alloys. All of the samples exhibit typical ferromagnetic curves (Fig. 6), the values of saturation magnetization (Ms), remanence (Mr), remanence-to-saturation ratio (Mr/Ms)and coercivity (Hc) are listed in Table 2. As shown in Fig. 6a, RGO–ZnNi5-1, RGO–ZnNi5-2 and RGO–ZnNi5-3 show the similar Ms values. However, the Ms value of the bare ZnNi5 particles (Fig. 6a) is slightly bigger than those of the RGO–ZnNi5 nanocomposites, which could be attributed to the bigger particle size.31 Because of similar shape anisotropy, these samples show similar Hc values (70–80 Oe) except that RGO–ZnNi5-3 has a relatively higher Hc value. The bigger Hc value may originate from its smaller size, which may change the magnetization reversal mechanism and lead to a higher coercivity.44
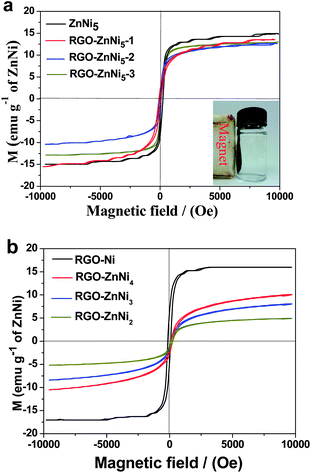 | ||
| Fig. 6 Magnetic hysteresis loops of the as-prepared samples measured at 300 K (the inset of (a) shows the magnetic separation of the composite). | ||
| Samples | M s (emu g−1 of ZnNi) | M r (emu g−1 of ZnNi) | M r/Ms | H c (Oe) |
|---|---|---|---|---|
| ZnNi5 | 14.95 | 3.02 | 0.20 | 80.45 |
| RGO–Ni | 16.10 | 5.10 | 0.32 | 115.30 |
| RGO–ZnNi5-1 | 12.70 | 2.35 | 0.19 | 78.23 |
| RGO–ZnNi5-2 | 12.54 | 1.75 | 0.18 | 75.03 |
| RGO–ZnNi5-3 | 12.67 | 2.31 | 0.18 | 90.45 |
| RGO–ZnNi4 | 9.95 | 1.42 | 0.14 | 67.54 |
| RGO–ZnNi3 | 7.84 | 0.69 | 0.09 | 60.11 |
| RGO–ZnNi2 | 4.70 | 0.50 | 0.11 | 55.48 |
The magnetic hysteresis loops of the composites with different Zn/Ni molar ratios are shown in Fig. 6b. The Ms value of Ni in RGO–Ni nanocomposite is calculated to be 16.10 emu g−1 at T = 300 K, which is much lower than the reported value of bulk Ni (55 emu g−1).45 This may be attributed to the small particle size of Ni nanoparticles in the composites, which can decrease the effective magnetic moment of Ni.46 With the increasing of Ni content in the RGO–ZnNi composites, the corresponding Ms and Hc values increase, which is in agreement with the reported FeNi alloys.47 Meanwhile, there are no big change of the smaller Mr/Ms values at T = 300 K for all the samples (all smaller than 0.32), suggesting that the samples have similar soft magnetic characteristics.48 Such RGO–ZnNi composites with soft-magnet behavior would have potential application in drug delivery, bioseparation, water treatment, and magnetic resonance imaging applications. In addition, due to the smaller size, the nanoparticle catalyst is generally hard to be recycled. However, our RGO–ZnNi nanocomposites with considerable Ms values make them easily be recollected from various dispersions by an external magnet (inset of Fig. 6a).
3.3. Catalytic properties of RGO–ZnNi nanocomposites
To quantitatively evaluate the catalytic activity of the as-synthesized nanocomposites, the reduction of 4-NP by NaBH4 was employed as a model reaction, which could be easily monitored by time-dependent UV-vis absorption spectra (Fig. S2† and Fig. S3†). It is well known that 4-NP exhibits a strong absorption peak at 400 nm in alkaline solution.49,50 As the reduction reaction proceeds, the intensity of the absorption peak at 400 nm gradually decreases. Meanwhile, new peaks at 300 nm from 4-AP appear with their intensity increasing with reaction time, indicating the gradual conversion of 4-NP to 4-AP.51 Among these RGO–ZnNi nanocomposites, RGO–ZnNi5-2 shows the highest catalytic activity, with which the reaction finished within 8 min (Fig. S2a†), while the RGO–ZnNi2 shows the lowest catalytic activity and cannot achieve the full reduction of 4-NP even with a reaction time of 50 min (Fig. S2d†). Moreover, compared with RGO–ZnNi5, the bare ZnNi5 shows much slower catalytic process with more than 50 min to finish the reaction (Fig. S3d†).Pseudo first order kinetics is often applied for the evaluation of rate constants in this reaction.52 In the reaction system, the ratio of Ct to C0 (Ct/C0) (Ct and C0 are the concentration of 4-NP at time t and 0, respectively) was measured from the ratio of the absorbances (At/A0) at 400 nm. Good linear correlations of ln (At/A0) versus time t are observed for various tested catalysts (Fig. S2f† and S3f†). The apparent rate constants (kapp), obtained from the slopes of the linearly fitted plots of ln (At/A0) − t, are provided in Table 3. The sample of RGO–ZnNi5-2 has the highest kapp value. The kapp values exhibit that the catalytic activities of RGO–ZnNi nanocomposites increase in the following order: RGO–ZnNi2 < RGO–ZnNi3 < RGO–ZnNi4 < RGO–ZnNi5, that is, the catalytic activities increase with the increasing Ni/Zn molar ratio in the ZnNi alloys. This is reasonable because metal Ni, as a commercial catalyst for the reduction of 4-NP, possesses much better catalytic activity than metal Zn.53 In addition, it is well-known that the catalytic activity of a nanoparticle is strongly dependent on its size.54,55 Usually, a decrease of the metal particle size leads to an increase in the fraction of low coordination metal sites such as vertices and edges on their surface, which can facilitate the reduction of 4-NP.56 As mentioned above, the size of ZnNi alloys nanocrystals on RGO sheets decreases with the increasing Ni/Zn molar ratio, which also contributes to the increasing catalytic activity. However, RGO–Ni with higher Ni content and smaller particle size shows lower catalytic activity than RGO–ZnNi5, which could be ascribed to the synergetic chemical coupling effects between Zn and Ni components in RGO–ZnNi5.10
| Samples | RGO–ZnNi5-1 | RGO–ZnNi5-2 | RGO–ZnNi5-3 | RGO–ZnNi4 | RGO–ZnNi3 | RGO–ZnNi2 | RGO–Ni | RGO | ZnNi5 |
|---|---|---|---|---|---|---|---|---|---|
| k app (min−1) | 0.1768 | 0.2354 | 0.1887 | 0.0841 | 0.0647 | 0.0481 | 0.1202 | 0.0118 | 0.0475 |
| R 2 | 0.9981 | 0.9502 | 0.9848 | 0.9911 | 0.9971 | 0.9985 | 0.9970 | 0.9750 | 0.9993 |
In comparison with bare ZnNi5 (kapp = 0.0475 min−1), the catalytic activities of RGO–ZnNi5 are much higher (up to 5 times that of bare ZnNi5). The rate constants obtained here are comparable to that of Au-based nanoparticles and GO/SnO2/Pt.57,58 As shown in Table 3, the RGO shows poor catalytic activity and the kapp value is only 0.0118 min−1. We consider that the higher catalytic activities of RGO–ZnNi5 than bare ZnNi5 could be attributed to the following two sides. One side, RGO plays an important role in the enhanced catalytic activity. The RGO sheets with higher adsorption ability towards 4-NP via π–π stacking interactions would provide a drive for enriching 4-NP molecules to the catalyst, ZnNi5 nanoparticles.52,59 On the other hand, ZnNi5 nanoparticles formed on the RGO sheets have much smaller sizes than bare ZnNi5 particles, which also improves the catalytic activity of RGO–ZnNi5.
It was found that a suitable loading amount of ZnNi5 is also crucial for optimizing the catalytic activity of RGO–ZnNi5 nanocomposites. As shown in Table 3, the catalytic activities of the samples with different loading amount of ZnNi5 increase in the following order: RGO–ZnNi5-1 < RGO–ZnNi5-3 < RGO–ZnNi5-2. The difference in catalytic activity can be attributed to the different loading amounts and particle sizes of ZnNi5 on RGO. It can be seen that with the increase of ZnNi5 loading amount, the catalytic activity increased at first and then decreased. That is reasonable that the catalytic activity increases with the increase of ZnNi5 loading amount within a certain range because of the increase in the catalytically active sites. However, when the ZnNi5 loading amount is further increased over its optimum value, the catalytic performance deteriorates, which may be due to the bigger particle size. As evidenced by TEM images in Fig. 5, RGO–ZnNi5-1 with the highest ZnNi5 loading amount shows much bigger particles size than RGO–ZnNi5-2, which would result in lower catalytic activity of RGO–ZnNi5-1 (kapp = 0.1768 min−1) than RGO–ZnNi5-2 (kapp = 0.2354 min−1). Therefore, the composition (Ni/Zn molar ratio), the particle size of ZnNi alloys and the synergetic chemical coupling effects between the two metal components all exert an important influence on the resulting catalytic activities of the RGO–ZnNi nanocomposites.10
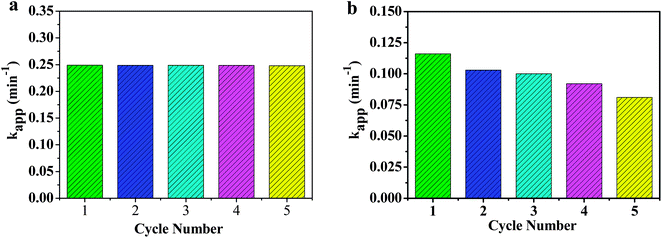
Catalysts based on noble metal nanoparticles often suffer from poisoning by the reaction product during the catalytic process.60,61 Thus, the improvement of the catalytic stability is the key priorities for practical applications. In order to evaluate the catalytic stability of RGO–ZnNi nanocomposites, the representative samples of RGO–ZnNi5-2 and RGO–Ni were tested for reusability in the reduction of 4-NP by NaBH4 for five times and the kapp values for the successive five cycles is shown in Fig. 7. It can be seen that the catalytic efficiency of RGO–ZnNi5-2 has no loss after five cycles, indicating the excellent stability of RGO–ZnNi5-2 catalyst (Fig. 7a) against poisoning by the product of the reaction. In contrast, RGO–Ni (Fig. 7b) catalyst performs a slight decrease in the kapp values and lost its catalytic activity over the course of each round of reaction gradually. These results show that the introduction of Zn into Ni can effectively improve the catalytic stability of RGO–Ni. From the experimental results, it is concluded that the RGO–ZnNi nanocomposites are excellent catalysts with characters of high efficiency, low cost and magnetic separation for the reduction of 4-NP into 4-AP, which endow the possibility of their practical applications.58,62,63
4. Conclusions
In summary, the RGO–ZnNi nanocomposites have been synthesized by in situ growth of ZnNi alloy nanocrystals accompanied by a co-reduction process. The size and morphology of ZnNi alloy on RGO sheets can be tuned by simply changing initial metal ion concentration and molar ratio of Zn2+ to Ni2+ in the EG solution. These RGO–ZnNi nanocomposites show ferromagnetic behavior and composition dependent magnetic properties. Especially, the RGO–ZnNi nanocomposites show excellent catalytic activity and extraordinary stability towards the reduction of 4-NP by NaBH4 with apparent rate constant as high as 0.2354 min−1, which is comparable to that of noble metal catalysts. Furthermore, the composites are much cheaper and could be easily magnetically separated for the reuse. It is expected that the RGO–ZnNi nanocomposites may find practical applications in catalysis and related areas.Acknowledgements
The authors are grateful for financial support from the National Natural Science Foundation of China (nos. 51272094, 51072071 and 51102117) and the Specialized Research Fund for the Doctoral Program of Higher Education of China (no. 20123227110018).Notes and references
- M. Kamigaito, T. Ando and M. Sawamoto, Chem. Rev., 2001, 101, 3689–3745 CrossRef CAS PubMed.
- D. S. Wang and Y. D. Li, Adv. Mater., 2011, 23, 1044–1060 CrossRef CAS PubMed.
- K. Kim, K. L. Kim and S. J. Lee, Chem. Phys. Lett., 2005, 403, 77–82 CrossRef CAS PubMed.
- A. F. Lee, C. J. Baddeley, C. Hardacre, R. M. Ormerod and R. M. Lambert, J. Phys. Chem., 1995, 99, 6096–6102 CrossRef CAS.
- R. Ferrando, J. Jellinek and R. L. Johnston, Chem. Rev., 2008, 108, 845–910 CrossRef CAS PubMed.
- X. Q. Huang, E. B. Zhu, Y. Chen, Y. J. Li, C. Y. Chiu, Y. X. Xu, Z. Y. Lin, X. F. Duan and Y. Huang, Adv. Mater., 2013, 25, 2974–2979 CrossRef CAS PubMed.
- Z. D. Pozun, S. E. Rodenbusch, E. Keller, K. Tran, W. J. Tang, K. J. Stevenson and G. Henkelman, J. Phys. Chem. C, 2013, 117, 7598–7604 CAS.
- M. Blosi, S. Albonetti, A. L. Costa, N. Sangiorgi and A. Sanson, Chem. Eng. J., 2013, 215, 616–625 CrossRef PubMed.
- P. H. Zhang, Y. M. Sui, G. J. Xiao, Y. N. Wang, C. Z. Wang, B. B. Liu, G. T. Zou and B. Zou, J. Mater. Chem. A, 2013, 1, 1632–1638 CAS.
- M. Raula, M. H. Rashid, S. Lai, M. Roy and T. K. Mandal, ACS Appl. Mater. Interfaces, 2012, 4, 878–889 CAS.
- K. R. Sriraman, S. Brahimi, J. A. Szpunar and S. Yue, J. Appl. Electrochem., 2013, 43, 441–451 CrossRef CAS.
- M. G. Hosseini, H. A. Sorkhabi and H. A. Ghiasvand, Surf. Coat. Technol., 2008, 202, 2897–2904 CrossRef CAS PubMed.
- M. E. Soares, C. A. C. Souza and S. E. Kuri, Surf. Coat. Technol., 2006, 201, 2953–2959 CrossRef CAS PubMed.
- M. Friedrich, D. Teschner, A. K. Gericke and M. Armbruster, J. Phys. Chem. C, 2012, 116, 14930–14935 CAS.
- J. Park, W. Kim, C. Suh and S. Kim, Met. Mater. Int., 2012, 18(2), 237–241 CrossRef CAS PubMed.
- C. V. Loricera, P. Castano, A. Infantes-Molina, I. Hita, A. Gutierrez, J. M. Arandes, J. L. G. Fierro and B. Pawelec, Green Chem., 2012, 14, 2759–2770 RSC.
- X. Q. Huang, E. B. Zhu, Y. Chen, Y. J. Li, C. Y. Chiu, Y. X. Xu, Z. Y. Lin, X. F. Duan and Y. Huang, Adv. Mater., 2013, 25, 2974–2979 CrossRef CAS PubMed.
- A. Peigney, C. Laurent, E. Flahaut, R. R. Bacsa and A. Rousset, Carbon, 2001, 39, 507–514 CrossRef CAS.
- X. W. Liu, J. J. Mao, P. D. Liu and X. W. Wei, Carbon, 2011, 49, 477–483 CrossRef CAS PubMed.
- H. Zhang, X. J. Lv, Y. M. Li, Y. Wang and J. H. Li, ACS Nano, 2010, 4, 380–386 CrossRef CAS PubMed.
- F. Ji, Y. L. Li, J. M. Feng, D. Su, Y. Y. Wen, Y. Feng and F. Hou, J. Mater. Chem., 2009, 19, 9603–9607 Search PubMed.
- T. Kuilla, S. Bhadra, D. H. Yao, N. H. Kim, S. Bose and J. H. Lee, Prog. Polym. Sci., 2010, 35, 1350–1375 CrossRef CAS PubMed.
- Y. Zhang, L. Q. Ren, S. R. Wang, A. Marathe, J. Chaudhuri and G. G. Li, J. Mater. Chem., 2011, 21, 5386–5391 RSC.
- Z. Y. Ji, G. X. Zhu, X. P. Shen, H. Zhou, C. M. Wu and M. Wang, New J. Chem., 2012, 36, 1774–1780 RSC.
- J. Jasinski and V. Leppert, Chem. Mater., 2008, 20, 6389–6395 CrossRef.
- C. V. Rao, C. R. Cabrera and Y. Ishikawa, J. Phys. Chem. C, 2011, 115, 21963–21970 Search PubMed.
- Y. Shin, I. T. Bae, B. W. Arey and G. J. Exarhos, J. Phys. Chem. C, 2008, 112, 4844–4848 CAS.
- S. Bai, X. P. Shen, G. X. Zhu, Z. Xu and J. Yang, CrystEngComm, 2012, 14, 1432–1438 RSC.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- Z. Y. Ji, X. P. Shen, M. Z. Li, H. Zhou, G. X. Zhu and K. M. Chen, Nanotechnology, 2013, 24, 115603 CrossRef PubMed.
- Z. Y. Ji, X. P. Shen, G. X. Zhu, H. Zhou and A. H. Yuan, J. Mater. Chem., 2012, 22, 3471–3477 RSC.
- X. W. Wei, X. M. Zhou, K. L. Wu and Y. Chen, CrystEngComm, 2011, 13, 1328–1332 RSC.
- M. J. Hu, B. Lin and S. H. Yu, Nano Res., 2008, 1, 303–313 CrossRef CAS.
- C. Z. Zhu, S. J. Guo, Y. X. Fang and S. J. Dong, ACS Nano, 2010, 4, 2429–2437 CrossRef CAS PubMed.
- A. C. Ferrari and P. Robertson, Phil. Trans. R. Soc. Lond. A, 2004, 362, 2477–2512 CrossRef CAS PubMed.
- J. C. Charlier, P. C. Eklund, J. Zhu and A. C. Ferrari, Carbon Nanotubes, 2008, 111, 673–709 CrossRef PubMed.
- A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401 CrossRef CAS.
- A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef PubMed.
- M. A. Pimenta, G. Dresselhaus, M. S. Dresselhaus, L. G. Cancado, A. Jorio and R. Saito, Phys. Chem. Chem. Phys., 2007, 9, 1276–1290 RSC.
- Z. Y. Huang, H. H. Zhou, C. H. Li, F. Y. Zeng, C. P. Fu and Y. F. Kuang, J. Mater. Chem., 2012, 22, 1781–1785 RSC.
- Y. X. Xu, H. Bai, G. W. Lu, C. Li and G. Q. Shi, J. Am. Chem. Soc., 2008, 130, 5856–5857 CrossRef CAS PubMed.
- S. Park, J. H. An, I. W. Jung, R. D. Piner, D. Richard, S. J. An, X. S. Li, A. Velamakanni and R. S. Ruoff, Nano Lett., 2009, 9, 1593–1597 CrossRef CAS PubMed.
- S. Bai, X. P. Shen, G. X. Zhu, M. Z. Li, H. T. Xi and K. M. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 2378–2386 CAS.
- X. W. Wei, G. X. Zhu, Y. J. Liu, Y. H. Ni, Y. Song and Z. Xu, Chem. Mater., 2008, 20, 6248–6253 CrossRef CAS.
- G. X. Zhu, X. W. Wei and S. Jiang, J. Mater. Chem., 2007, 17, 2301–2306 RSC.
- S. H. Wu and D. H. Chen, J. Colloid Interface Sci., 2003, 259, 282–286 CrossRef CAS.
- H. Q. Wu, Y. J. Cao, P. S. Yuan, H. Y. Xu and X. W. Wei, Chem. Phys. Lett., 2005, 406, 148–153 CrossRef CAS PubMed.
- D. Ung, G. Viau, C. Ricolleau, F. Warmont, P. Gredin and F. Fiéver, Adv. Mater., 2005, 17, 338–344 CrossRef CAS.
- S. Praharaj, S. Nath, S. K. Ghosh, S. Kundu and T. Pal, Langmuir, 2004, 20, 9889–9892 CrossRef CAS PubMed.
- J. Zeng, Q. Zhang, J. Y. Chen and Y. N. Xia, Nano Lett., 2010, 10, 30–35 CrossRef CAS PubMed.
- M. H. Rashid, R. R. Bhattacharjee, A. Kotal and T. K. Mandal, Langmuir, 2006, 22, 7141–7143 CrossRef CAS PubMed.
- Z. Y. Ji, X. P. Shen, J. L. Yang, G. X. Zhu and K. M. Chen, Appl. Catal., B, 2014, 144, 454–461 CrossRef CAS PubMed.
- A. L. Wang, H. B. Yin, H. H. Lu, J. J. Xue, M. Ren and T. S. Jiang, Langmuir, 2009, 25, 12736 CrossRef CAS PubMed.
- B. M. Min and C. M. Friend, Chem. Rev., 2007, 107, 2709–2724 CrossRef CAS PubMed.
- J. Lee, J. C. Park and H. Song, Adv. Mater., 2008, 20, 1523–1528 CrossRef CAS.
- R. Narayanan and M. A. E. Sayed, J. Am. Chem. Soc., 2003, 125, 8340–8347 CrossRef CAS PubMed.
- C. Z. Zhu, P. Wang, L. Wang, L. Han and S. J. Dong, Nanoscale, 2011, 3, 4376–4382 RSC.
- J. Li, C. Y. Liu and Y. Liu, J. Mater. Chem., 2012, 22, 8426–8430 RSC.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed.
- F. H. Lin and R. A. Doong, J. Phys. Chem. C, 2011, 115, 6591–6598 CAS.
- D. Jana, A. Dandapat and G. De, Langmuir, 2010, 26, 12177–12184 CrossRef CAS PubMed.
- P. Wang, L. Han, C. Z. Zhu, Y. M. Zhai and S. J. Dong, Nano Res., 2011, 4, 1153–1162 CrossRef CAS PubMed.
- P. Zhang, C. L. Shao, Z. Y. Zhang, M. Y. Zhang, J. B. Mu, Z. C. Guo and Y. C. Liu, Nanoscale, 2011, 3, 3357–3363 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S3. See DOI: 10.1039/c3ra45210g |
| This journal is © The Royal Society of Chemistry 2014 |