Water-soluble highly fluorinated graphite oxide†
Ondřej
Jankovský
a,
Petr
Šimek
a,
David
Sedmidubský
a,
Stanislava
Matějková
b,
Zbyněk
Janoušek
b,
Filip
Šembera
b,
Martin
Pumera
c and
Zdeněk
Sofer
*a
aInstitute of Chemical Technology, Department of Inorganic Chemistry, 166 28 Prague 6, Czech Republic. E-mail: zdenek.sofer@vscht.cz; Fax: +420 22431-0422
bInstitute of Organic Chemistry and Biochemistry AS CR, v.v.i., Flemingovo nam. 2., 166 10 Prague 6, Czech Republic. E-mail: Stanislava.Matejkova@uochb.cas.cz; Fax: +420 220183578
cDivision of Chemistry & Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore, 637371, Singapore. E-mail: pumera@ntu.edu.sg; Fax: +65 6791-1961
First published on 8th November 2013
Abstract
Water-soluble highly fluorinated graphite oxide is a promising candidate for applications in biosensing and for fluorescent probes due to its variable fluorescence properties. We report on a simple process for the preparation of a fluorinated graphite oxide (FGO). This process is based on fluorination of graphite oxide (GO) in a fluorine atmosphere at an elevated temperature and pressure. We used two different GO precursors, which were prepared by Staudenmaier and Hummers methods. The method of GO synthesis has a strong influence on the concentration of fluorine in the obtained product. The mechanism of GO fluorination is associated with the presence of reactive groups, mostly epoxides, and is accompanied by etching of graphite oxide. Our analyses highlighted that the FGO prepared by Hummers method contains a significantly higher amount of bounded fluorine and can be used as a starting material for the synthesis of chemically reduced fluorine doped graphene. Water soluble fluorinated graphene can be easily processed in aqueous solutions to create hydrophilic particles and films with tunable fluorescence properties.
Introduction
Graphene is a carbon allotrope with a single planar sheet of sp2 hybridized bonded carbon atoms, which are densely packed in a honeycomb crystal lattice,1 graphene has been studied due to its outstanding physical and chemical properties. These properties can be influenced by various chemical modifications, such as oxidation, hydrogenation2,3 or halogenations.4 Such modifications can lead to fabrication of materials for electrochemical sensing,5 energy storage,6 optoelectronics or photonics.7Fluorinated graphene, fluorographene, is a semiconductor; therefore it can be used for electronic applications such as LEDs.8 By means of the Density Functional Theory (DFT) band structure calculations the relative stability and electronic properties of fluorinated derivatives of graphene were studied.9 The structural and the electronic properties of fluorographene nanoribbons were investigated by first principle calculations.10 Fluorinated graphene was prepared most frequently by exfoliation of graphite fluoride11,12 or by the reaction with atomic fluorine, which was formed by the decomposition of XeF2.13 Other possibility to obtain fluorinated graphene is to fluorinate graphite oxide (GO) by hydrogen fluoride at elevated temperature14 or electrochemically by the electrochemical intercalation of graphite flakes.15 Plasma treated fluorinated graphenes were successfully synthetized.16 Yang et al.17 reported the preferential and reversible fluorination of SF6 plasma treated n-layer (or few layer) graphene. Ionic liquids were also successfully used to exfoliate commercially available fluorinated graphite to fabricate fluorinated graphene.18
All these methods are in general complicated and they take place under extreme conditions. Moreover, these methods usually led to the synthesis of strongly hydrophobic fluorinated graphene, which is difficult to disperse in water based solvents. We focused on the development of simple and cost-effective method of fluorinated graphite oxide fabrication, which can be produced in large-scale. This method is based on the fluorination of GO in fluorine atmosphere at elevated temperatures and pressures. Our method of graphite oxide fluorination can be easily transferred to the large scale industrial production. Synthesized fluorinated graphite oxides (FGOs) were extensively characterized by physical and chemical methods. Our results show that the different GO precursors have significant influence on the degree of fluorination and on the structure and properties of the obtained fluorinated graphite oxide. The strongly fluorescent FGOs are hydrophilic and are suitable for application in biosensing and biomedical applications.19,20
Experimental section
Graphite oxides were prepared, similarly to Staudenmaier method21 and Hummers22 method, from pure graphite microparticles (2–15 μm, 99.9995%, from Alfa Aesar). Sulfuric acid (98%), nitric acid (68%), fuming nitric acid (>98%), potassium chlorate (98%), potassium permanganate (98%), hydrogen peroxide (30%), hydrochloric acid (37%), silver nitrate (99.5%), barium nitrate (99.5%) and N,N-dimethylformamide (DMF) were provided by Penta, Czech Republic. A mixture of 20 vol% F2/80 vol% N2 obtained from Solvay, Germany, was used for fluorination. Argon and nitrogen both of 99.999% purity were delivered by SIAD. Phosphate buffer solution (PBS), 0.05 M, was prepared from potassium hydrogen phosphate and potassium dihydrogen phosphate obtained from Penta, Czech Republic. Deionized water (16.8 Mohm) was used for buffer preparation.Graphite oxide prepared by the Staudenmaier method will be denoted ST-GO hereinafter. 87.5 mL of concentrated sulfuric acid and 27 mL of fuming nitric acid were added to a reaction flask containing a magnetic stir bar. The mixture was then subsequently cooled to 0 °C and 5 g of graphite was added. The mixture was vigorously stirred to avoid agglomeration and to obtain a homogeneous dispersion. While keeping the reaction flask at 0 °C, 55 g of potassium chlorate was slowly added to the mixture in order to avoid a sudden increment in temperature and the formation of explosive chlorine dioxide gas. Upon the complete dissolution of potassium chlorate, the reaction flask was then loosely capped to allow the evolving gas to escape and the mixture was continuously stirred vigorously for 96 h at room temperature. On completion of the reaction, the mixture was then poured into 3 L of deionized water and decanted. Graphite oxide was then redispersed in HCl (5%) solutions to remove sulfate ions and repeatedly centrifuged and redispersed in deionized water until a negative reaction on chloride and sulfate ions (with AgNO3 and Ba(NO3)2, respectively) was achieved. Graphite oxide slurry was then dried in a vacuum oven at 50 °C for 48 h before use.
The second graphite oxide was synthesized similarly to the Hummers method and is notated as HU GO. 5 g of graphite and 2.5 g of sodium nitrate were stirred with 115 mL of concentrated sulfuric acid. The mixture was then cooled on 0 °C. With vigorous stirring, 15 g of potassium permanganate was then added over a period of two hours. In the subsequent four hours, the reaction mixture was allowed to reach room temperature before being heated to 35 °C for 30 min. The reaction mixture was then poured into a flask containing 250 mL of deionized water and heated to 70 °C for 15 minutes. The mixture was then poured into 1 L of deionized water. The unreacted potassium permanganate and manganese dioxide were removed by the addition of 3% hydrogen peroxide. The reaction mixture was then allowed to settle and decanted. The graphite oxide obtained was then purified by repeated centrifugation and redispersed in deionized water until a negative reaction on sulfate ion (with Ba(NO3)2) was achieved. Graphite oxide slurry was then dried in a vacuum oven at 50 °C for 48 h before use.
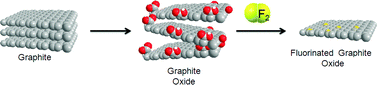
In the next step, the prepared GOs were fluorinated in the PTFE autoclave connected to a fluorine line.23 An autoclave with two small Teflon reactors charged with GOs was slowly evacuated and filled with N2 twice to remove oxygen and once more evacuated. Next, the autoclave was slowly pressurized by 20% F2/N2 (v/v) to 3.2 bar and heated to 60 °C for six days. Afterwards the autoclave was cooled down to room temperature and the gas was removed under vacuum. The autoclave was then filled by N2 twice and opened. Fluorinated graphite oxides are named ST-FGO and HU-FGO according to the method of preparation.
Combustible elemental analysis (CHNS-O) was performed with a PE 2400 Series II CHNS/O Analyzer (Perkin Elmer, USA). In CHN operating mode (the most robust and interference free mode), the instrument employs a classical combustion principle to convert the sample elements to simple gases (CO2, H2O and N2). The PE 2400 analyzer performs automatically combustion and reduction, homogenization of product gases, separation and detection. A microbalance MX5 (Mettler Toledo) was used for precise weighing of samples (1.5–2.5 mg per single sample analysis). The accuracy of CHN determination is better than 0.30% abs. Internal calibration is performed using N-phenyl urea.
For the measurement of fluorine concentration the samples were decomposed for analysis according to Schöniger method. The exact amount of sample (about 2 mg) was wrapped in an ash free paper, burned in pure oxygen atmosphere and leached out with deionized water and total ionic strength adjustment buffer (TISAB) was added. The concentration of fluorine was determined by potentiometric measurement with an ion-selective electrode (ISE).
The morphology of graphite oxide before and after fluorination was investigated by scanning electron microscopy using Tescan Lyra dual beam microscope with FEG electron source. The elemental composition and mapping was performed with energy dispersive spectroscopy (EDS) analyzer X-MaxN with 20 mm2 SDD detector from Oxford instruments and software package AZtecEnergy. For the measurement the powder sample was placed on a carbon conductive tape. The observation and EDS measurement was performed using 10 kV electron beam. Transmission electron microscopy was performed by means of scanning transmission electron (STEM) detector on Tescan Lyra dual beam microscope operated in both bright field and dark field mode and an accelerating voltage of 30 kV. The specimens for STEM were prepared by dropping the aqueous suspension (1 mg mL−1) on a copper carbon grid (1 μL, 200 mesh grid).
X-ray powder diffraction data were collected at room temperature with an X'Pert PRO θ–θ powder diffractometer with parafocusing Bragg–Brentano geometry using CuKα radiation (λ = 1.5418 Å, U = 40 kV, I = 30 mA). Data were scanned with an ultrafast detector X'Celerator over the angular range 5–80° (2θ) with a step size of 0.0167° (2θ) and a counting time of 20.32 s step−1. Data evaluation was performed in the software package HighScore Plus.
High resolution X-ray photoelectron spectroscopy (XPS) was performed with a ESCAProbeP (Omicron Nanotechnology Ltd, Germany) spectrometer using a monochromatic aluminum X-ray radiation source (1486.7 eV). A wide-scan survey of all elements was performed, with subsequent high-resolution scans of the C 1s core level spectra and F 1s. Relative sensitivity factors were used in evaluation of the carbon-to-oxygen (C/O) ratios from the survey spectra. Samples were applied onto conductive carrier made from freshly cut indium block. The electron gun was used to eliminate sample charging during measurement (1–5 V).
Samples were analyzed by simultaneous DTA a TG analysis (STA) Linseis PT 1600 using a low temperature furnace from 0 °C to 500 °C. 2 mg of sample were weighted on digital balances with an accuracy of 0.01 mg, put into Al2O3 crucible, and reweighted after the process finished confirming TG results. Heating rate was set to 5 K min−1 and the measurement was carried out in a dynamic argon atmosphere with flow rate 50 mL min−1.
An inVia Raman microscope (Renishaw, England) was used for Raman spectroscopy in backscattering geometry with a CCD detector. For the measurement, a Nd-YAG laser was used (532 nm, 50 mW) with 50x magnification objective. Instrument calibration was achieved with a silicon reference which gives a peak position at 520 cm−1 and a resolution of less than 1 cm−1. In order to avoid a radiation damage of studied material the laser power, used for this measurement, was in the range of 50 mW to 50 nW. Samples were suspended in deionized water in concentration 1 mg mL−1 and ultrasonicated for 10 minutes. The suspension was deposited on a small piece of silicon wafer and dried.
For AFM measurement the samples were suspended in water (1 mg mL−1), ultrasonicated for 10 minutes and dropped on freshly cleaved mica substrate. These measurements were carried out on NT-MTD Ntegra Spectra from NT-MDT in a tapping mode.
The FT-IR measurement was performed on FTIR spectrometer NICOLET 6700 (Thermo Scientific, USA). Diamond ATR crystal and DTGS detector were used for the measurements in the range of 4000–400 cm−1.
The particle size and zeta potential was determined using Malvern Zetasizer Nano-ZS in the Dynamic light scattering (DLS) regime for particle size distribution. The Zeta potential was measured by electrophoretic light scattering. DPSS laser (50 mW, 532 nm) was used as a light source and the signal was detected by avalanche photodiode. For the measurement of zeta potential the sample was dispersed by ultrasonication (10 min/15 W) in 50 mM phosphate buffer solution. The measurement was performed in polystyrene cuvets with gold coated electrodes.
The electrochemical characterization was performed by cyclic voltametry using potentiostat Interface 1000 (Gamry, USA) with three electrode set-up. Glassy carbon working electrode (GC), platinum auxiliary electrode (Pt) and Ag/AgCl reference electrode were obtained from Gamry. For the cyclovoltametric measurements fluorinated graphite oxide was dispersed in DMF (1 mg mL−1) and 1 μL was evaporated on glass carbon working electrode. All given potentials are referred to Ag/AgCl reference electrode.
Results and discussion
Two graphite oxides (GOs) were prepared by either the Staudenmaier or Hummers method. These GO samples were fluorinated in the PTFE autoclave at elevated pressure and temperature in a fluorine/nitrogen (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) atmosphere. Fluorinated graphite oxides were named ST-FGO and HU-FGO according to the method of preparation (see Scheme 1). For more details see experimental section. We characterized material properties in all steps to gain understanding of the process.
4 ratio) atmosphere. Fluorinated graphite oxides were named ST-FGO and HU-FGO according to the method of preparation (see Scheme 1). For more details see experimental section. We characterized material properties in all steps to gain understanding of the process.
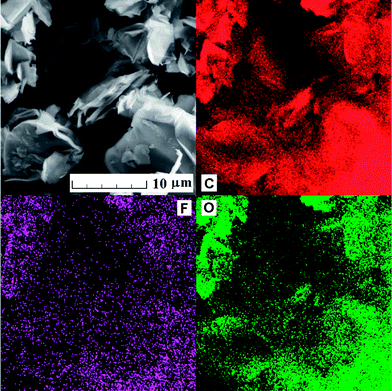
First, the morphology of GO and FGO was studied by scanning electron microscopy (SEM). The ST-FGO sample shows typical platelet structure which is usually observed on graphite oxide sample. The HU-FGO sample reveals a different morphology with wrinkled plate structure. This observation is related to the fluorination procedure accompanied with side sheet etching. This conclusion was confirmed by observation of separated fluorinated graphite oxide sheets by AFM and STEM. The morphology of fluorinated graphite oxide was observed by SEM: HU-FGO is shown on Fig. 1 and ST-FGO on Fig. 2. The distribution of fluorine and main elements like carbon and oxygen was measured by EDS. The obtained results from element distribution mapping can be seen on the same figures. On the HU-FGO sample, a very uniform distribution of fluorine within the sample is observed. This observation indicates that the reaction of fluorine is performed on the edges and on the body of the graphite oxide flakes. The average concentration of fluorine within HU-FGO sample measured by EDS is 11.1 ± 0.7 wt%. In this sample the typical impurities introduced during graphite oxidation procedure can be also observed. The concentration of typical impurities measured by EDS is 2.2 wt% S, 0.4 wt% Mn, 0.3 wt% K and 0.1% Cl. The concentration of fluorine within the ST-FGO sample is much lower. This is related to differences in composition and concentration of oxygen functional groups and will be in detail explained later. However, the distribution of fluorine within the sample is also quite uniform and the distribution of fluorine scales the concentration map of oxygen and carbon. The concentration of fluorine measured by EDS is 1.9 ± 0.3 wt% of F. In the ST-FGO sample the elements introduced by formation of starting ST-GO material can be also identified. The main impurities are sulfur (1.0 wt%) and chlorine (0.2 wt%).
The graphite oxide samples before and after the fluorination procedure were also investigated by STEM. The HU-GO and HU-FGO samples before and after fluorination procedure observed in dark field and bright field mode are shown on Fig. 3. The ST-GO sample and ST-FGO samples observed in bright field and dark field mode are shown on Fig. 4. On the image of HU-GO a single layer of graphite oxide with slight wrinkling due to the presence of defects can be seen. The fluorination procedure of HU-GO clearly leads to a smaller particle size and higher concentration of defects. These defects are mainly located on the rims of graphite oxide sheets. This corresponds to the observations obtained by AFM discussed in the next paragraph. A slightly different morphology can be observed on ST-GO sample. Due to the lower degree of oxidation we can observe particles composed of single and also few layer graphite oxides. The particle size is also smaller when compared to the HU-GO sample. Similarly to HU-GO, the fluorination procedure results in a significant reduction of particle size. The observations obtained by STEM are in good agreement with the data obtained by other methods like AFM and particle size distribution measured by dynamic light scattering (DLS).
 | ||
| Fig. 3 Graphite oxide prepared by Hummers method before and after fluorination procedure (HU-GO and HU-FGO). The left images are observed in dark field mode, right images are in bright field mode. | ||
The structure of GO and FGO was investigated by the X-ray diffraction analysis. When comparing ST-GO and HU-GO diffractograms (see Fig. 5), peaks are much broader in the former sample. This effect is due to smaller particle size of the resulting GO which is obvious from FWHM of (101) diffraction line at 2θ = 42.4° corresponding to an AB plane particle size. In HU-GO there is a (002) diffraction line corresponding to GO at 11.5°, while this peak is shifted by ∼1° to higher angle in the case of ST-GO. Using the Bragg equation we calculated the distance between graphene layers as 7.08 Å and 7.69 Å for ST-GO and HU-GO, respectively, while in graphite the interlayer distance is 3.40 Å. This result corresponds to the knowledge of oxidation, which is well known and correlates with other analyses. The second broad peak in ST-GO at 25.2° is close to the graphite (002) diffraction line. This peak is entirely missing in HU-GO. In the XRD diffraction pattern, the presence of the (002) diffraction line is an indication of the degree of oxidation of the graphite oxides where an absence of the (002) line around 26.4° 2θ implies a complete oxidation of graphite accompanied by a significant increase of graphite interlayer spacing. By comparing the ST-GO and ST-FGO it is evident that the ST-FGO sample contains smaller particles due to etching of AB plane sides by fluorine. In HU-FGO the main peak (at 10.7°) is split and shifted to lower values of 2θ in comparison to HU-GO. This effect is apparently caused by strong C–F bond formation and further expansion of interlayer spacing due to the repulsive effect of highly electronegative fluorine.
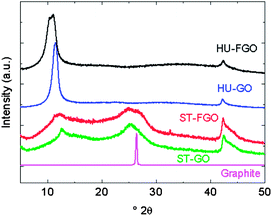 | ||
| Fig. 5 X-ray diffraction of graphite oxides prepared by Staudenmaier and Hummers method (ST-GO and HU-HO), fluorinated graphite oxides (ST-FGO and HU-FGO) in comparison to the pure graphite. | ||
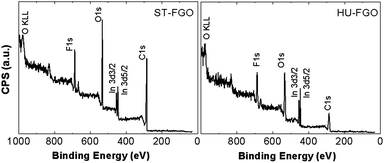
Consequently, we used high resolution X-ray photoelectron spectroscopy to gain insight into the chemical composition. The XPS survey spectra of both FGOs are presented in Fig. 6, where the peaks of C 1s, O 1s and F 1s are clearly visible. For the survey spectra of HU GO and ST GO sample see ESI (Fig. S1†). Energies of these peaks are at 284.5 eV, 533.0 and 685.9 eV, respectively. They display the compositions and the functional groups. The amounts of oxygen-containing groups on the surfaces of both samples were determined by using XPS wide scan spectra. The composition of both fluorinated samples was calculated based on C 1s, O 1s and F 1s peak areas. ST-FGO contains (in at%) 74.3% C, 19.1% O and 6.6% F, whereas the composition of HU-FGO is 50.6% C, 35.1% O and 14.3% F. Both spectra contain also indium peak originating from the indium carrier. XPS results showed successful fluorination of both samples, but the higher content of fluorine was achieved in HU-FGO sample. This is seemingly a consequence of higher oxidation degree allowing fluorine to substitute more functional groups by the formation of C–F bonds. The remaining traces of sulfur are related to the method of GO synthesis where concentrated sulfuric acid is used.
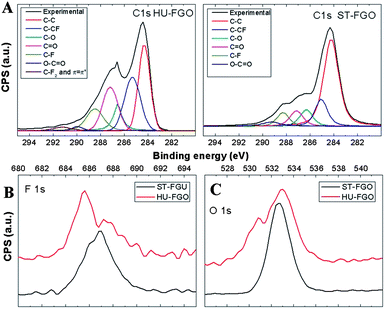
Detailed high resolution XPS (Fig. 7a) spectra of C 1s exhibit a single peak at 284.5 eV with an asymmetrical tail at higher energies. Careful curve fitting was performed on both C 1s spectrum to quantitatively differentiate the seven different carbon stages in ST-FGO: the C–C (284.5 eV), the C–CF (285.4 eV), the C–O of alcohol/ether groups (286.6 eV), the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of carbonyl groups (287.2 eV), C–F (288.47 eV), O–C
O of carbonyl groups (287.2 eV), C–F (288.47 eV), O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of carboxylic acid/ester groups (289.9 eV) and the π–π* interaction (291.2 eV). The position of π–π* interaction is overlapping with the signal of carbon in the form of C–F2 (ref. 14). The high resolution spectra of HU-FGO contain significant differences compared to ST-FGO related to large differences in composition of functional groups and also to higher degree of fluorination. High intensity of C–CF bond located at 285.4 eV is in good agreement with the data obtained from survey spectra indicating higher concentration of fluorine. The high concentration of carbonyl groups originates from much higher degree of oxidation of the HU-GO sample. A part of these groups remains unaffected during fluorination procedure. Lower concentration of functional groups in starting ST-GO led to a lower concentration of fluorine in fluorinated ST-GO. From these observations we can conclude that the fluorination procedure of graphite oxide is strongly related to the presence of functional groups in graphite oxide reacting with fluorine under the subsequent formation of C–F bonds. The weak band located around 291.2 eV is attributed to the presence of C–CF2 bonds. These bonds can be mostly formed on the edges of graphite layer. This peak can be observed only on HU-FGO sample. The higher reactivity of carbon atoms on graphene edges and in the vicinity of large defects led to CF2 groups formation on the rim of graphene. This process is also most probably accompanied by intensive etching of graphene backbone. This conclusion is based on our observation of HU-FGO sample by AFM. The relative abundance of the pertinent functional groups is shown in Table 1. The high resolution spectra of C 1s for HU-GO and ST-GO samples are provided in Supplementary Information (Fig. S2†).
O of carboxylic acid/ester groups (289.9 eV) and the π–π* interaction (291.2 eV). The position of π–π* interaction is overlapping with the signal of carbon in the form of C–F2 (ref. 14). The high resolution spectra of HU-FGO contain significant differences compared to ST-FGO related to large differences in composition of functional groups and also to higher degree of fluorination. High intensity of C–CF bond located at 285.4 eV is in good agreement with the data obtained from survey spectra indicating higher concentration of fluorine. The high concentration of carbonyl groups originates from much higher degree of oxidation of the HU-GO sample. A part of these groups remains unaffected during fluorination procedure. Lower concentration of functional groups in starting ST-GO led to a lower concentration of fluorine in fluorinated ST-GO. From these observations we can conclude that the fluorination procedure of graphite oxide is strongly related to the presence of functional groups in graphite oxide reacting with fluorine under the subsequent formation of C–F bonds. The weak band located around 291.2 eV is attributed to the presence of C–CF2 bonds. These bonds can be mostly formed on the edges of graphite layer. This peak can be observed only on HU-FGO sample. The higher reactivity of carbon atoms on graphene edges and in the vicinity of large defects led to CF2 groups formation on the rim of graphene. This process is also most probably accompanied by intensive etching of graphene backbone. This conclusion is based on our observation of HU-FGO sample by AFM. The relative abundance of the pertinent functional groups is shown in Table 1. The high resolution spectra of C 1s for HU-GO and ST-GO samples are provided in Supplementary Information (Fig. S2†).
| ST-FGO | HU-FGO | |
|---|---|---|
| C–C | 29.42 | 54.75 |
| C–CF | 24.93 | 14.85 |
| C–O | 9.34 | 9.46 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
19.74 | 9.21 |
| C–F | 12.63 | 7.51 |
O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
1.65 | 4.22 |
| CF2 + π = π* | 2.29 | 0.00 |
The detailed spectra of F 1s are shown on Fig. 7b. We can observe the differences between the fluorinated samples in the high resolution spectra. The ST-FGO sample shows a single maximum on energy 686.9 eV with a slight broadening to the lower energy. We can conclude that in the ST-FGO the single C–F bond is dominantly present. In the case of HU-FGO the twinning of F 1s peak is observed with the first maximum at 685.6 eV and the second maximum at 687.3 eV. This indicates a different type of fluoride carbon bond or a different surroundings of C–F bond. This observation is supported by the data obtained by FT-IR spectroscopy indicating the occurrence of acyl fluorides and also by high resolution C 1s spectra corroborating the presence of other types of fluorine based functional groups like C–CF2 groups.7,24
The high resolution O 1s spectra of fluorinated GO are shown on Fig. 7c. Large differences in the shape of spectra originate from the method of GO synthesis. Typical twining of O 1s signal in HU-FGO reveals maxima at 530.9 eV and 533.3 eV. This twining of O 1s is associated with the presence of carbonyl group and with carbon–oxygen double bond (like C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –O– at 533.3 eV) and C–O single bond like in hydroxyl group at 530.9 eV.25 On the other hand, in ST-FGO only one maximum at 532.8 eV can be observed which is mostly associated with –O– bond.26,27 The high resolution O 1s spectra of GO are shown in ESI (Fig. S3†).
O and –O– at 533.3 eV) and C–O single bond like in hydroxyl group at 530.9 eV.25 On the other hand, in ST-FGO only one maximum at 532.8 eV can be observed which is mostly associated with –O– bond.26,27 The high resolution O 1s spectra of GO are shown in ESI (Fig. S3†).
The composition (wt%) of the major elements measured by the combustible elemental analysis in ST-GO sample was as follows: 73.7 wt% C, 1.00 wt% H and 0.30 wt% N. The remaining 25.0 wt% is to be mainly attributed to oxygen. The C/O ratio in ST-GO is ∼2.95 implying a relatively low degree of oxidation. Hummers method of synthesizing graphite oxide (HU-GO) gave an elemental composition 47.6 wt% C, 2.40 wt% H and 0.07 wt% N and the remaining 49.93 wt% of oxygen, with a C/O ratio ∼0.95. Hummers method provides a graphite oxide with extremely high degree of oxidation. The concentration of functional groups in HU-GO is three times higher than in ST-GO and it is strongly related to the subsequent reactivity of graphite oxide. The elemental composition of both samples significantly changed after the fluorination process. The resulting concentration of elements in ST-FGO was 70.8 wt% C, 1.16 wt% H and 0.00 wt% N. The 19.9 wt% completing the sum are represented by fluorine and oxygen. After the sample combustion according to Schöniger method, the concentration of fluorine measured by ISE gives 0.0 wt% of fluorine, however 6.6 at% was observed by XPS measurement. This result can be explained in terms of high thermal stability of C–F bonds leading to incomplete combustion of the sample and no free fluoride ions are observed in solution after sample decomposition according to Schöniger method.
In HU-FGO the composition after the fluorination was defined by elemental combustion analysis in CHN mode as 41.7 wt% C, 2.53 wt% H and 0.0 wt% N. The remaining 55.77 wt% consist of oxygen and fluorine. The concentration of fluorine obtained by sample combustion according to Schöniger method and subsequent measurement with ISE give 1.2 wt% F. This result is also much lower compared to data obtained by XPS. The C–F bonds are very strong and FGOs are thus thermally stable, hence, the detected fluorine obviously originates from a small portion of thermally unstable functional groups such as carbonyl fluorides. This effect explains why the composition of ST-GO and ST-FGO has changed despite the zero detection of fluorine. The concentration of functional group, which can produce labile fluorine group (mainly –COOH), is very low in ST-GO and we are not able to observe any free fluoride ion by ISE. On the base of fluorine measurements with ISE, we can also conclude that this method of fluorine concentration measurement is most probably selective to the reactive functional groups. This opinion is supported by FT-IR measurement discussed in following paragraphs.
The results of STA analysis showed different behavior during the heating before and after the fluorination. While in ST-GO there are two peaks with maxima at 188 °C and 218 °C, in ST-FGO there is only one peak at 222 °C (see Fig. 8a). This is likely caused by the substitution effect of less stable epoxy groups by fluorine, which shifts the maximum of the thermal effect to higher temperatures. TG curves showed that ST-GO lost 22.9% of its weight during the heating, whereas ST-FGO lost 29.1%.
STA of HU-GO and HU-FGO (Fig. 8b) revealed a completely different thermal exfoliation behavior. While in HU-GO there is one broad peak at 205 °C, in HU-FGO there are two peak maxima at 193 °C and 238 °C, respectively. The second peak is shifted more than 30 °C to higher temperatures. This effect can be associated with the decomposition of fluorine containing groups, such as acyl fluorides and other thermally labile fluorine containing functional groups. Because of the higher oxidation level both samples lost more weight during the heating. HU-GO lost 51.7% and HU-FGO lost even 58.7%. These results indicate that some fluorine is also evolved during the exfoliation process.
The Raman spectra of all samples (Fig. 9) show two major bands: the D-band at ∼1350 cm−1 that corresponds to the sp3 defects in the sp2 graphene plane and the G-band at ∼1600 cm−1 that corresponds to the sp2 bonded carbon in the graphitic materials.28 There are two minor bands at higher wavenumbers. 2D band appears at ∼2690 cm−1 and D′′ band at ∼2920 cm−1.29 The slight shift of G-band after fluorination of HU-GO to higher wavenumber is related to the formation of C–F bond. By comparing ST-GO and HU-GO the different intensity of background is noticeable. Much higher background of HU-GO sample is caused by large fluorescence of the sample itself as discussed later. The same effect can be observed by comparing HU-GO and HU-FGO. C–F groups generate very strong fluorescence background in the latter one.
The ratio between the D and G band intensities (D/G ratio) can be used as an indication of the degree of disorder in a carbon structure. D/G ratios of ST-GO and HU-GO were found close to ∼1 (0.98 for ST-GO and 1.01 for HU-GO) and remained almost unchanged after the fluorination (1.03 for ST-FGO and 1.02 for HU-FGO). From this observation we can conclude that the fluorination mechanism is not based on direct formation of C–F bond by reaction of fluorine with carbon, but the presented functional groups play a key role in the formation of C–F bond. The mechanism of fluorination is based on epoxide ring opening and substitution of oxygen functionalities with fluorine. This mechanism led to only slight changes in D/G ratio. In addition, the average crystallite size (La) can also be directly calculated using the D/G ratio based on equation30
| La = 2.4 × 10−10 × λlaser4 × IG/ID | (1) |
The IG/ID is the ratio of the intensities of the D and G bands respectively and λlaser refers to the laser wavelength (nm) used in the measurement of the Raman spectrum, i.e. λlaser = 532 nm. The fluorination mechanism did not cause any significant crystallite size decrease and the reduction of structural quality. The crystallite size of ST-GO is 19.7 nm and ST-FGO 18.6 nm. In the case of HU-GO we obtained comparable data, the size of HU-GO is 19.1 nm and HU-FGO 20.7 nm. The etching of GO took place preferably on the edges of individual sheets which was confirmed by observation with AFM. The etching of sheet surface occurred only partially in locations with high concentration of defects and led to higher population of holes and other defects in graphene network (see AFM data).
Subsequently, FT-IR analyses were carried out to investigate the presence of specific bond types in the GOs and FGOs. As seen from Fig. 10, prominent peaks corresponding to O–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–O–C stretching modes were observed at approximately υ = 3200, 1720, and 1050 cm−1.31 On HU-GO sample we can also observe weak vibration band located around 950 cm−1 which is commonly attributed to the organic peroxides on graphite oxide.32 Large peak at υ = 1600 cm−1 corresponds to the sp2 vibration of graphene backbone.33 A closer examination of the FTIR spectra revealed a larger peak of C
O, and C–O–C stretching modes were observed at approximately υ = 3200, 1720, and 1050 cm−1.31 On HU-GO sample we can also observe weak vibration band located around 950 cm−1 which is commonly attributed to the organic peroxides on graphite oxide.32 Large peak at υ = 1600 cm−1 corresponds to the sp2 vibration of graphene backbone.33 A closer examination of the FTIR spectra revealed a larger peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching. In the case of ST-FGO no significant changes was observed. This is related to the low degree of fluorination and high absorption coefficient of the investigated material. In the case of HU-FGO we can observe significant shift and changes in the spectral region of 900 to 1500 cm−1. The increase of absorption in the region of 1100–1200 cm−1 is related to the formation of C–F bonds.34 On the other hand we can observe a suppression of intensity for absorption band related to the C–O vibration at 1340 cm−1 and 950 cm−1.35 This indicates these oxygen functional groups are necessary for direct fluorination of graphite oxide and they are substituted by fluorine in order to form C–F bond. These reactions take place on most reactive groups like epoxides and peroxides. However, slight changes in –O–H vibration band intensity indicate that a part of hydroxyl groups on graphite oxide is substituted by fluorine. Slight shift of C
O stretching. In the case of ST-FGO no significant changes was observed. This is related to the low degree of fluorination and high absorption coefficient of the investigated material. In the case of HU-FGO we can observe significant shift and changes in the spectral region of 900 to 1500 cm−1. The increase of absorption in the region of 1100–1200 cm−1 is related to the formation of C–F bonds.34 On the other hand we can observe a suppression of intensity for absorption band related to the C–O vibration at 1340 cm−1 and 950 cm−1.35 This indicates these oxygen functional groups are necessary for direct fluorination of graphite oxide and they are substituted by fluorine in order to form C–F bond. These reactions take place on most reactive groups like epoxides and peroxides. However, slight changes in –O–H vibration band intensity indicate that a part of hydroxyl groups on graphite oxide is substituted by fluorine. Slight shift of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration band of carboxylic acid (from 1715 cm−1 for HU-GO to 1740 cm−1 for HU-FGO) led us to conclusion that carboxyl fluorides are formed. This also supports the measurement of fluorine concentration with ISE where only minor part of fluorine was observed. This functional group is highly reactive and easily undergoes thermal and hydrolytic decomposition.
O vibration band of carboxylic acid (from 1715 cm−1 for HU-GO to 1740 cm−1 for HU-FGO) led us to conclusion that carboxyl fluorides are formed. This also supports the measurement of fluorine concentration with ISE where only minor part of fluorine was observed. This functional group is highly reactive and easily undergoes thermal and hydrolytic decomposition.
Results of the particle size analysis are plotted on Fig. 11a. It shows that the fluorination process leads to a significant reduction of particle size. This observation indicates that the fluorination process is accompanied by the strong etching of graphene layers. The particle size measured by dynamic light scattering represents an average size approximated by the sphere. We admit relatively large errors for the particle size determination, however, we would like to show the obvious tendency during the fluorination. These results are consistent with XRD analysis and they are, moreover, confirmed by AFM measurement. The zeta potential of GO samples before and after fluorination procedure was measured in order to investigate the aqueous suspension stability and hydrophilic properties. The distribution of zeta potential is shown on Fig. 11b. In order to increase the conductivity of suspension the measurement was performed in 50 mM PBS. The knowledge of zeta potential of particles suspended in solvent is important in order to investigate the stability of such colloidal suspension. Usually the values lower the −40 mV or higher then +40 mV, respectively, are necessary to obtain stable aqueous colloidal suspension. The introduction of fluorine in the graphene framework is usually accompanied with the formation of highly hydrophobic material. In the case of graphite oxide fluorination under mild condition we observed completely different behavior. The zeta-potential was −39.3 mV and −46.3 mV for ST-GO and ST-FGO, respectively. A similar trend was obtained for HU-GO sample where the zeta potential was −28.5 mV and −31.2 mV for HU-GO and HU-FGO, respectively. This is related to the mechanism of GO fluorination which was discussed in the previous paragraphs. The fluorination is mainly performed on the oxygen functionalities like epoxide ring opening. This reaction led to formation of C–F bonds and simultaneous formation of hydroxyls groups. On the base of presented results we can conclude that compared to fluorination of graphene, the fluorination of graphite oxide led to the formation of hydrophilic fluorinated graphite oxide which can form stable aqueous suspensions.
 | ||
| Fig. 11 (A) Particle size analysis measured by dynamic light scattering (DLS), (B) distribution of zeta potential before and after fluorination procedure. | ||
AFM images of both FGOs are shown on Fig. 12a. The images from AFM confirm our observation from previous analyses that graphite oxide is etched on the GO sheets side during the fluorination procedure. In the case of HU-GO with much higher degree of oxidation we can clearly observe separated fluorinated graphite oxide sheets with defects in graphene backbone related to the action of fluorine. The thickness of single layer sheet measured by AFM is 0.8 nm. The ST-GO with lower degree of oxidation is also etched from the side of graphene sheets which was confirmed by dynamic light scattering. The ST-FGO exhibits a structure of few layer graphene with a thickness about 10 nm and a significant reduction of flake size. The height profile of ST-FGO and HU-FGO particles can be seen on Fig. 12b.
 | ||
| Fig. 12 (A) Morphology of fluorinated graphite oxides ST-FGO and HU-FGO observed by AFM. The height profile can be seen on (B). | ||
The results obtained from FT-IR characterization and high resolution XPS spectra of C 1s are supported by electrochemical characterization. The cyclic voltametry was performed on samples in the range of 0 to −2.5 V in 50 mM PBS solution on GO material before and after the fluorination procedure. The obtained data are shown on Fig. 13. On the HU-GO we can clearly observe reduction of oxygen functional groups with maxima at the potentials −1.63 V and −2.05 V. After fluorination procedure this double peak completely disappears and only a weak signal is observed from −1.05 V. This indicates that the oxygen functionalities like peroxides and epoxides were abstracted by nucleophilic attack of fluorine molecules and subsequent formation of C–F bonds.36 The cyclovoltamogram of ST-GO is completely different and we can observe a reduction of oxygen functionalities with peak maximum at −1.05 V. After the fluorination procedure we observe two reduction peaks with maxima at −0.68 V and −1.37 V. Their amplitude is again much lower compared to ST-GO indicating significant changes in the composition of oxygen functional group on GO surface.
 | ||
| Fig. 13 Electrochemical characterization of GO before and after fluorination. The measurement was performed in 50 mM PBS. | ||
The photoluminescence measurements confirmed assumptions inferred from Raman spectra: while ST-samples showed only weak luminescence, this effect increased more than one order of magnitude in HU-samples (See Fig. 14). C–F bonds are indeed responsible for luminescence increment, since both fluorinated samples show stronger luminescence than the non-fluorinated samples. The strong luminescence of fluorinated graphene and its derivatives has been reported by several authors.7,30 The strong band centered at ∼730 nm is present in all samples except for HU-GO, where this band is close to ∼780 nm. The slight blue shift of HU-FGO (from ∼780 nm to ∼730 nm) is caused by the fluorination process of GO. However, this effect is insignificant in ST-FGO due to lower fluorine concentration. Another weak band is present in all samples at ∼630 nm.
Conclusions
Graphite oxides and fluorinated graphite oxides were characterized by an extensive spectrum of analytical techniques in order to understand the mechanism of fluorination. The presence of functional groups is important for the formation of C–F bonds. By means of XPS, the concentration of fluorine after the fluorination procedure was 6.6 wt% and 14.3 wt% for Staudenmaier GO and Hummers GO, respectively. The amount of fluorine detected by SEM-EDS was smaller, 1.9 wt% and 11.1 wt%. The presence of fluorine was measured by XPS and titration with an ion selective electrode after sample decomposition. The formation of C–F bonds was also confirmed by FT-IR spectroscopy. The changes observed in FT-IR spectra and results obtained from electrochemical measurement clearly indicate that the oxygen functional groups are necessary for the reaction of graphite oxide with fluorine under such mild conditions. During the reaction with fluorine etching from the graphite oxide sheets side also occurs which was confirmed by AFM and dynamic light scattering measurement. The presence of epoxide and peroxides groups on graphite oxide is crucial for fluorination of graphite oxide and their high concentration can lead to graphite oxide with higher degree of fluorination or even to graphene fluoride.In this paper we presented a simple and scalable method for the fabrication of fluorescent and water-soluble fluorinated graphite oxide. This method is based on the fluorination of GO in fluorine atmosphere at elevated temperatures and pressures. This procedure can be easily adopted for industrial production of these materials.
Acknowledgements
Research was supported by Specific University Research (MSMT no. 20/2013). Z.J. and F.Š. acknowledge the support of the Institute of Organic Chemistry and Biochemistry, Academy of Science of the Czech Republic, Project No. RVO61388963. M. P. thanks to NAP (NTU) fund.Notes and references
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- D. C. Elias, R. R. Nair, T. M. G. Mohiuddin, S. V. Morozov, P. Blake, M. P. Halsall, A. C. Ferrari, D. W. Boukhvalov, M. I. Katsnelson, A. K. Geim and K. S. Novoselov, Science, 2009, 323, 610–613 CrossRef CAS PubMed.
- A. Y. Eng, H. L. Poh, F. Šaněk, M. Maryško, S. Matějková, Z. Sofer and M. Pumera, ACS Nano, 2013, 7, 5930–5939 CrossRef CAS PubMed.
- H. L. Poh, P. Šimek, Z. Sofer and M. Pumera, Chem.–Eur. J., 2013, 19, 2655–2662 CrossRef CAS PubMed.
- M. Pumera, A. Ambrosi, A. Bonanni, E. L. K. Chng and H. L. Poh, TrAC, Trends Anal. Chem., 2010, 29, 954–965 CrossRef CAS.
- M. Pumera, Energy Environ. Sci., 2011, 4, 668–674 CAS.
- K. J. Jeon, Z. Lee, E. Pollak, L. Moreschini, A. Bostwick, C. M. Park, R. Mendelsberg, V. Radmilovic, R. Kostecki and T. J. Richardson, ACS Nano, 2011, 5, 1042–1046 CrossRef CAS PubMed.
- R. R. Nair, W. Ren, R. Jalil, I. Riaz, V. G. Kravets, L. Britnell, P. Blake, F. Schedin, A. S. Mayorov, S. Yuan, M. I. Katsnelson, H. Cheng, W. Strupinski, L. G. Bulusheva, A. V. Okotrub, I. V. Grigorieva, A. N. Grigorenko, K. S. Novoselov and A. K. Geim, et al. , Small, 2010, 6, 2877–2884 CrossRef CAS PubMed.
- A. N. Enyashin and A. L. Ivanovskii, Chem. Phys. Lett., 2012, 545, 78–82 CrossRef CAS.
- S. Tang and S. Zhang, J. Phys. Chem. C, 2011, 115, 16644–16651 CAS.
- R. Zbořil, A. B. Bourlinos, T. A. Steriotis, A. K. Stubos, V. Georgakilas, K. Šafářová, D. Jančík, C. Trapalis and M. Otyepka, Small, 2010, 6, 2885–2891 CrossRef PubMed.
- S. H. Cheng, K. Zou, F. Okino, H. R. Gutierrez, A. Gupta, N. Shen, P. Eklund, J. Sofo and J. Zhu, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 205435 CrossRef.
- J. T. Robinson, J. S. Burgess, C. E. Junkermeier, S. C. Badescu, T. L. Reinecke, F. K. Perkins, M. K. Zalalutdniov, J. W. Baldwin, J. C. Culbertson and P. E. Sheehan, Nano Lett., 2010, 10, 3001–3005 CrossRef CAS PubMed.
- Z. Wang, J. Wang, Z. Li, P. Gong, X. Liu, L. Zhang, J. Ren, H. Wang and S. Yang, Carbon, 2012, 50, 5403–5410 CrossRef CAS.
- M. Bruna, B. Massessi, C. Cassiago, A. Battiato, E. Vittone, G. Speranza and S. Borini, J. Mater. Chem., 2011, 21, 18730–18737 RSC.
- S. B. Bon, L. Valentini, R. Verdejo, J. L. Garcia Fierro, L. Peponi, M. A. Lopez-Manchado and J. M. Kenny, Chem. Mater., 2009, 21, 3433–3438 CrossRef.
- H. Yang, M. Chen, H. Zhou, C. Qiu, L. Hu, F. Yu, W. Chu, S. Sun and L. Sun, J. Phys. Chem. C, 2011, 115, 16844–16848 CAS.
- H. Chang, J. Cheng, X. Liu, J. Gao, M. Li, J. Li, X. Tao, F. Ding and Z. J. Zheng, Chem.–Eur. J., 2011, 17, 8896–8903 CrossRef CAS PubMed.
- G. Eda, Y.-Y. Lin, C. Mattevi, H. Yamaguchi, H.-A. Chen, I.-S. Chen, C.-W. Chen and M. Chhowalla, Adv. Mater., 2010, 22, 505–509 CrossRef CAS PubMed.
- B. Wang, J. R. Sparks, H. R. Gutierrez, F. Okino, Q. Hao, Y. Tang, V. H. Crespi, J. O. Sofo and J. Zhu, Appl. Phys. Lett., 2010, 97, 141915 CrossRef.
- L. Staudenmaier, Ber. Dtsch. Chem. Ges., 1898, 31, 1481–1487 CrossRef CAS.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- M. Valášek, F. Šembera, M. J. Hughes, I. Stibor, Z. Janoušek and J. Michl, Efficient Preparation of Fluorine Compounds, ed. H. W. Roesky, 2012, John Wiley & Sons, Hoboken, NJ, p. 22 Search PubMed.
- S. Kwon, J.-H. Ko, K.-J. Jeon, Y.-H. Kim and J. Y. Park, Nano Lett., 2012, 12, 6043–6048 CrossRef CAS PubMed.
- D. Yang, A. Velamakanni, G. Bozoklu, S. Park, M. Stoller, R. D. Piner, S. Stankovich, I. Jung, D. A. Field, A. C. Ventrice Jr and R. S. Ruoff, Carbon, 2009, 47, 145–152 CrossRef CAS.
- C. Mattevi, G. Eda, S. Agnoli, S. Miller, K. A. Mkhoyan, O. Celik, D. Mastrogiovanni, G. Granozzi, E. Garfunkel and M. Chhowalla, Adv. Funct. Mater., 2009, 19, 2577–2583 CrossRef CAS.
- O. Akhavan, Carbon, 2010, 48, 509–519 CrossRef CAS.
- D. R. Dreyer, R. S. Ruoff and C. W. Bielawski, Angew. Chem., Int. Ed., 2010, 49, 9336–9344 CrossRef CAS PubMed.
- M. S. Dresselhaus, A. Jorio, M. Hofmann, G. Dresselhaus and R. Saito, Nano Lett., 2010, 10, 751–758 CrossRef CAS PubMed.
- L. G. Cançado, K. Takai, T. Enoki, M. Endo, Y. A. Kim, H. Mizusaki, A. Jorio, L. N. Coelho, R. Magalhães-Paniago and M. A. Pimenta, Appl. Phys. Lett., 2006, 88, 163106 CrossRef.
- D. W. Lee, L. V. De Los Santos, J. W. Seo, L. L. Felix, A. Bustamante, J. M. Cole and C. H. W. Barnes, J. Phys. Chem. B, 2010, 114, 5723–5728 CrossRef CAS PubMed.
- P. Guo, H. Song and X. Che, J. Mater. Chem., 2010, 20, 4867–4874 RSC.
- Y. Si and E. T. Samulski, Nano Lett., 2008, 8, 1679–1682 CrossRef CAS PubMed.
- L. Pu, Y. Ma, W. Zhang, H. Hu, Y. Zhou, Q. Wang and Ch. Pei, RSC Adv., 2013, 3, 3881–3884 RSC.
- C. Hontoria-Lucas, A. J. Lopez-Peinado, J. de D. Lopez-Gonzalez, M. L. Rojas-Cervantes and R. M. Martın-Aranda, Carbon, 1995, 33, 1585–1592 CrossRef CAS.
- E. L. K. Chng and M. Pumera, Chem.–Asian J., 2011, 6, 2899–2901 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra45183f |
| This journal is © The Royal Society of Chemistry 2014 |