Efficient preparation of some new 1-carbamato-alkyl-2-naphthols using N-halo reagents in neutral media†
Ardeshir
Khazaei
*a,
Fatemeh
Abbasi
a and
Ahmad Reza
Moosavi-Zare
*b
aFaculty of Chemistry, Bu-Ali Sina University, Hamedan 6517838683, Iran. E-mail: Khazaei_1326@yahoo.com; Fax: +98 8118257407
bDepartment of Chemistry, University of Sayyed Jamaleddin Asadabadi, Asadabad, 6541835583, Iran. E-mail: moosavizare@yahoo.com
First published on 13th November 2013
Abstract
A new and simple procedure for the synthesis of some new 1-carbamato-alkyl-2-naphthol derivatives via one-pot three-component condensation of arylaldehydes, 2-naphthol, and benzylcarbamate in the presence of catalytic amounts of 1,3,5-trichloro-1,3,5-triazinane-2,4,6-trione (TCCA) and 1,3-dichloro-5,5-dimethylhydantoin (DCDMH) under solvent-free conditions is described.
Multi-component reactions (MCRs) play an interesting role in combinatorial chemistry due to the ability to synthesize target products with greater efficiency and atom economy by forming structural complexity in a single step from three or more reactants. Moreover, MCRs offer advantages of simplicity and synthetic efficiency over conventional chemical reactions.1–3
1-Amidoalkyl-2-naphthols and 1-carbamatoalkyl-2-naphthols are of importance as they can be easily converted to 1-aminoalkyl-2-naphthol derivatives by hydrolysis, which are biologically important compounds. 1-Aminoalkyl-2-naphthols have been applied as hypotensive and bradycardiac agents.4,5 1-Amidoalkyl-2-naphthols can be also converted to 1,3-oxazine derivatives.6 Several pharmaceutical properties have been reported for 1,3-oxazines, e.g., antibiotic,7 antitumor,8 and analgesic activities.9
The synthesis of 1-amidoalkyl-2-naphthols can be carried out by multi-component condensation reaction of 2-naphthol, aromatic aldehydes, and acetonitrile or various amides in the presence of different catalysts such as p-toluenesulfonic acid,10 sulfamic acid/ultrasound,11 oxalic acid,12 Fe(HSO4)3,13 H3PMo12O40·xH2O/SiO2,14 trityl chloride,15,16 sulfonic acid functionalized imidazolium salt,17 iodine,18 silica-supported perchloric acid,19 NaHSO4·H2O,20 Bi(NO3)3·5H2O,21 supported ionic liquid catalyst (SILC),22 SnCl4·5H2O.23 However, some of the reported methods are associated with one or more of the following disadvantages such as low yield, long reaction time, toxic and corrosive solvent, strong acidic media, high reaction temperature (>100 °C) and using of toxic, corrosive, expensive and large amount catalysts. Because of the importance of these compounds, the improvement of a milder, faster, and more eco-friendly method accompanied with higher yields is still needed. Moreover, limited protocols have reported on the replacement of amide with benzylcarbamate.24–28
Trichloro-1,3,5-triazinane-2,4,6-trione (TCCA) (Fig. 1), an inexpensive, low toxicity, easily available reagent, has been widely used in organic transformations.29 1,3-dichloro-5,5-dimethylhydantoin (DCDMH) (Fig. 1) is a stable, low cost and commercially available heterocycle which has rarely used as a source of chlorine ion or radical in chlorination30,31 or oxidation reactions.32,33 But, as our knowledge, there are no reports of catalytic application of DCDMH and TCCA in the synthesis of 1-carbamato-alkyl-2-naphthols until now.
 | ||
| Fig. 1 The structure of trichloro-1,3,5-triazinane-2,4,6-trione (TCCA) and 1,3-dichloro-5,5-dimethylhydantoin (DCDMH). | ||
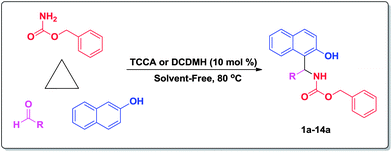
Herein, we were interested to examine the catalytic activity of DCDMH and TCCA as homogenous and neutral catalysts in the preparation of 1-carbamato-alkyl-2-naphthols by one-pot, three-component coupling of 2-naphthol, aromatic aldehydes, and benzylcarbamate (Scheme 1).
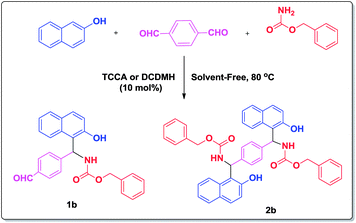
Moreover, TCCA and DCDMH can catalyze the synthesis of bis (1-carbamatoalkyl-2-naphthol)s (Scheme 2).
First of all, to optimize the reaction conditions (amount of catalysts and temperature), the reaction of 2-naphthol (1 mmol), benzaldehyde (1 mmol) and benzylcarbamate (1.1 mmol) was selected as a model reaction, and its behavior was studied in the presence of different amounts of catalysts (TCCA and DCDMH) under thermal solvent-free conditions. The results are summarized in Tables 1 and 2. As Tables 1 and 2, indicates that higher yield and shorter reaction time were obtained using 10 mol% of catalysts at 80 °C under solvent-free conditions.
To assess the efficiency and the scope of the organic catalyst in the preparation of 1-carbamato-alkyl-2-naphthols, the condensation of 2-naphthol with various arylaldehydes and benzylcarbamate was examined in the presence of 10 mol% of TCCA and DCDMH at 80 °C under solvent-free condition. The corresponding results are displayed in Table 3. As it can be seen in Table 3, the reactions were carried out efficiently within 2–35 min, and the most desired products were produced in high to excellent yields. Thus, TCCA and DCDMH are highly efficient, general and mild organic catalysts for the preparation of 1-carbamato-alkyl-2-naphthols. The influence of electron-releasing substituents, electron-withdrawing substituents and halogens on the aromatic ring of arylaldehydes on the reaction results was investigated. As Table 3 indicates, electron-withdrawing substituents and halogens had no significant effect on the yields, and arylaldehydes bearing these substituents were reacted faster than benzaldehyde (entries 2a–10a, products which were prepared using TCCA); however, electron-releasing groups decreased the yields and increased the reaction times (entries 12a–14a).
| Entry | R | Time (min)/Yielda (%) (TCCA) | Time (min)/Yielda (%) (DCDMH) | M.p. °C (Lit.) |
|---|---|---|---|---|
| a Isolated yield. | ||||
| 1a | C6H5 | 4/87 | 7/83 | 180–182 (180–182)27 |
| 2a | 2-NO2C6H4 | 2/93 | 7/88 | 198–200 (208–210)26 |
| 3a | 3-NO2C6H4 | 2/90 | 16/87 | 196–197 (200–202)26 |
| 4a | 4-NO2C6H4 | 3/92 | 4/90 | 200 (202–204)26 |
| 5a | 2-ClC6H4 | 3/89 | 5/81 | 214–216 (211–213)26 |
| 6a | 3-ClC6H4 | 4/84 | 5/80 | 183–185 |
| 7a | 4-ClC6H4 | 5/89 | 11/85 | 176–177 (178–180)26 |
| 8a | 2,4-diClC6H3 | 2/90 | 4/86 | 202–204 (208–210)26 |
| 9a | 4-FC6H4 | 3/84 | 16/79 | 202–203 |
| 10a | 4-BrC6H4 | 4/88 | 9/80 | 180–182 |
| 11a | 1-C10H7 | 10/80 | 18/76 | 214–217 |
| 12a | 4-CH3C6H4 | 15/60 | 30/58 | 152–154 |
| 13a | 2-OCH3C6H4 | 7/78 | 25/72 | 202–204 |
| 14a | 4-OCH3C6H4 | 30/50 | 35/47 | 165–167 |
Our method also worked well when 2-naphthol was reacted with bis-aldehyde (terephthaldehyde) and benzylcarbamate in the presence of 10 mol% of TCCA and DCDMH (Scheme 2). The use of 1 equiv. of 2-naphthol, 1 equiv. of terephthalaldehyde and 1.1 equiv. of benzylcarbamate in the reaction afforded 1-carbamato-alkyl-2-naphthol (1b) and bis(carbamato-alkyl-2-naphthol) (2b) in 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11% in 3 min and 77
11% in 3 min and 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18% in 19 min for TCCA and DCDMH, respectively. However, the condensation of 2 equiv. of 2-naphthol with 1 equiv. of the aldehyde and 2.2 equiv. of benzylcarbamate gave compounds 1b:2b in 67
18% in 19 min for TCCA and DCDMH, respectively. However, the condensation of 2 equiv. of 2-naphthol with 1 equiv. of the aldehyde and 2.2 equiv. of benzylcarbamate gave compounds 1b:2b in 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29% in 2 min and 61
29% in 2 min and 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35% in 17 min for TCCA and DCDMH, respectively. For the synthesis of bis(1-carbamatoalkyl-2-naphthol) (2b), increase the amount of catalyst to 20 mol%, due to two formyl groups in terephthaldehyde, did not improve the corresponded time and yield.
35% in 17 min for TCCA and DCDMH, respectively. For the synthesis of bis(1-carbamatoalkyl-2-naphthol) (2b), increase the amount of catalyst to 20 mol%, due to two formyl groups in terephthaldehyde, did not improve the corresponded time and yield.
 | ||
| Scheme 3 The plausible mechanism for the synthesis of 1-carbamatoalkyl-2-naphthols catalyzed by TCCA and DCDMH. | ||
To show the advantage, applicability and efficiency of TCCA and DCDMH over the reported catalysts for the synthesis of 1-carbamatoalkyl-2-naphthols from benzaldehyde, β-naphthol and benzylcarbamte, we have compared results and turn-over frequency (TOF = yield (%)/[reaction time (min) × mol% of catalyst]) of our catalysts with other reported catalysts. As shown in Table 4, TCCA and DCDMH can act as effective catalysts with respect to reaction times and yields of the obtained products.
| Catalyst | Catalyst (mol%) | Time (min) | Yielda (%) | TOF (min−1)b | Ref. |
|---|---|---|---|---|---|
| a Isolated yield. b Turn-over frequency. c Our work. d N-(4-sulfonic acid)butylpyridinium hydrogen sulfate. | |||||
| TCCA | 10 | 4 | 87 | 2.175 | —c |
| DCDMH | 10 | 7 | 83 | 1.185 | —c |
| Al(MS)3.4H2O | 2 | 36 | 85 | 1.180 | 26 |
| [(CH2)4SO3HPy][HSO4]d | 10 | 9 | 84 | 0.93 | 27 |
| HCl | 10 | 300 | 30 | 0.01 | —c |
| HCl | 20 | 300 | 35 | 0.005 | —c |
Since TCCA and DCDMH contain chlorine atoms which bonded to nitrogen atoms in heterocyclic ring. By in situ generation of Cl+ from the catalysts, the reaction is catalyzed. So, in a proposed reaction mechanism which supported by the literature,29,34–36 we suggest that aldehyde and catalysts (TCCA or DCDMH) produce intermediate I and II (Scheme 3). Then, 2-naphthol attacks to I and II to give intermediates III. Intermediate III converts to IV after tautomerisation. Finally, one molecule of benzylcarbamate attacks to IV and by removing one molecule H2O in this step, the corresponded product and catalyst are obtained.
In another investigation, to confirm that HCl could not produce from TCCA and DCDMH to catalyze the reaction, the model reaction was performed in the presence of hydrochloric acid (10% and 20%). As it shown of Table 4, indicates that TCCA and DCDMH were more effective than HCl to catalyze the reaction.
To prove the formation of intermediate I and II, benzaldehyde was reacted with catalysts at 80 °C, and then IR and UV spectra of the aldehydic functional group in the reaction mixture was compared with those in benzaldehyde as follows: IR (Nujol): υmax (cm−1) of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in benzaldehyde (1703) decreased to 1682 in the reaction mixture (Fig. 2).
O in benzaldehyde (1703) decreased to 1682 in the reaction mixture (Fig. 2).
UV spectra were another evidence to confirm of these forms (I and II). UV studies were performed in dichloromethane as solvent. The maximum of absorption for benzaldehyde appear at 243 nm and for TCCA at 234 nm and DCDMH at 231 nm. But λmax of the complexes of aldehyde and Cl+ which formed by adding TCCA and DCDMH to benzaldehyde was observed at 238 nm (Fig. 3).
 | ||
| Fig. 3 UV spectra of benzaldehyde, TCCA, DCDMH and complexes of benzaldehyde and Cl+ (I and II) at 80 °C in dichloromethane. | ||
These intermediates (I and II) act as activated carbonyl compounds and then react with 2-naphthol to give III, which reacts with benzylcarbamate to produce 1-carbamato-alkyl-2-naphthols. To investigate that the catalyst was completely recovered during the reaction and proceeded the synthesis of 1-carbamatoalkyl-2-naphthols; we have recorded the UV spectra of the catalyst and reaction mixture in dichloromethane as solvent respectively. We have exactly observed the maximum of absorption for the catalysts in UV spectrum of the reaction mixture. Based on, it is clear that TCCA and DCDMH was recovered unchanged after the completion of the reaction (Fig. 4) and the proposed mechanisms were acceptable.
 | ||
| Fig. 4 UV spectra of TCCA, DCDMH and mixture of reaction with TCCA and DCDMH at 80 °C in dichloromethane respectively. | ||
Conclusions
In conclusion, we have introduced a new method for the preparation of 1-carbamato-alkyl-2-naphthol derivatives via the one-pot three-component reaction of 2-naphthol with aromatic aldehydes and benzylcarbamate using trichloroisocyanuric acid (TCCA) and 1,3-dichloro-5,5-dimethylhydantoin (DCDMH) as homogenous organic catalysts at 80 °C under solvent-free and neutral conditions.37 Moreover, bis (1-carbamatoalkyl-2-naphthol)s have synthesized under these reaction conditions.38 The advantages of the presented method are efficiency, generality, high yield, short reaction time, cleaner reaction profile, and simplicity.Acknowledgements
The authors acknowledge to Bu-Ali Sina University Research Councils, Center of Excellence in Development of Chemistry Methods (CEDCM) and National Foundation of elites (BMN) for support of this work.Notes and references
- Multicomponent Reactions, ed. J. Zhu and H. Bienayme, Wiley, Weinheim, 2005 Search PubMed.
- A. Hasaninejad, A. Zare, M. Shekouhi and J. Ameri Rad, J. Comb. Chem., 2010, 12, 844 CrossRef CAS PubMed.
- A. Khazaei, M. A. Zolfigol, A. R. Moosavi-Zare, A. Zare, M. Khojasteh, Z. Asgari, V. Khakyzadeh and A. Khalafi-Nezhad, Catal. Commun., 2012, 20, 54 CrossRef CAS.
- H. R. Shaterian, H. Yarahmadi and M. Ghashang, Bioorg. Med. Chem. Lett., 2008, 18, 788 CrossRef CAS PubMed.
- A. Y. Shen, C. T. Tsai and C. L. Chen, Eur. J. Med. Chem., 1999, 34, 877 CrossRef CAS.
- M. Damodiran, N. P. Selvam and P. T. Perumal, Tetrahedron Lett., 2009, 50, 5474 CrossRef CAS.
- Y. Kusakabe, J. Nagatsu, M. Shibuya, O. Kawaguchi, C. Hirose and S. Shirato, J. Antibiot., 1972, 25, 44 CrossRef CAS PubMed.
- S. Renullard, L. I. Rebhun, G. A. Havic and S. M. Kupchan, Science, 1975, 189, 1002 Search PubMed.
- G. Y. Lesher and A. R. Surrey, J. Am. Chem. Soc., 1955, 77, 636 CrossRef CAS.
- M. M. Khodaei, A. R. Khosropour and H. Moghanian, Synlett, 2006, 6, 916 CrossRef.
- S. B. Patil, P. R. Singh, M. P. Surpur and S. D. Samant, Ultrason. Sonochem., 2007, 14, 515–518 CrossRef CAS PubMed.
- S. A. M. K. Ansari, J. N. Sangshetti, N. D. Kokare, P. S. Wakte and D. B. Shinde, Indian J. Chem. Technol., 2010, 17, 71 CAS.
- H. R. Shaterian, H. Yarahmadi and M. Ghashang, Bioorg. Med. Chem. Lett., 2008, 18, 788 CrossRef CAS PubMed.
- A. Zare, A. Hasaninejad, E. Rostami, A. R. Moosavi-zare, N. Pishahang, M. Roshankar, F. Khedri and M. Khedri, E.-J. Chem., 2010, 7, 1162 CrossRef CAS.
- A. Khazaei, M. A. Zolfigol, A. R. Moosavi-Zarea, A. Zare, A. Parhami and A. Khalafi-Nezhad, Appl. Catal., A, 2010, 386, 179 CrossRef CAS.
- A. Khazaei, M. A. Zolfigol, A. R. Moosavi-Zarea, F. Abi, A. Zare, H. Kaveh, V. Khakyzadeh, M. Kazem-Rostami, A. Parhami and H. Torabi-Monfared, Tetrahedron, 2013, 69, 212 CrossRef CAS.
- M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare, A. Zare and V. Khakyzadeh, Appl. Catal., A, 2011, 400, 70 CrossRef CAS.
- B. Das, K. Laxminarayana, B. Ravikanth and B. R. Rao, J. Mol. Catal. A: Chem., 2007, 261, 180 CrossRef CAS.
- H. R. Shaterian, H. Yarahmadi and M. Ghashang, Tetrahedron, 2008, 64, 1263 CrossRef CAS.
- H. R. Shaterian and H. Yarahmadi, ARKIVOC, 2008, 2, 105 Search PubMed.
- M. Wang, Y. Liang, T. T. Zhang and J. J. Gao, Chin. Chem. Lett., 2012, 23, 65 CrossRef CAS.
- D. A. Kotadia and S. S. Soni, J. Mol. Catal. A: Chem., 2012, 353–354, 44 CrossRef CAS.
- M. Wang, Y. Liang, T. T. Zhang and J. J. Gao, Chem. Nat. Compd., 2012, 48, 185 CrossRef CAS.
- H. R. Shaterian, A. Hosseinian and M. Ghashang, Tetrahedron Lett., 2008, 49, 5804 CrossRef CAS.
- H. R. Shaterian, K. Azizi and N. Fahimi, Chem. Sci. Trans., 2012, 1, 73 CrossRef CAS.
- Z. Song, X. Sun, L. Liu and Y. Cui, Res. Chem. Intermed., 2012, 39, 2123 CrossRef.
- N. Tavakoli-Hoseini, M. M. Heravi, F. F. Bamoharran and A. Davoodnia, Bull. Korean Chem. Soc., 2011, 32, 787 CrossRef CAS.
- A. Zare, T. Yousofi and A. R. Moosavi-Zare, RSC Adv., 2012, 2, 7988 RSC.
- L. Yang, E-J. Chem., 2012, 9, 2424 CrossRef CAS.
- Z. Xu, D. Zhang and X. Zou, Synth. Commun., 2006, 36, 255 CrossRef CAS.
- M. Skibinska, F. A. Zahn, A. Czarnocka and W. Lewenstein, Acta Pol. Pharm., 1969, 6, 25 CAS.
- A. Khazaei and A. Amini-Manesh, J. Chin. Chem. Soc., 2005, 52, 1017 CrossRef CAS.
- A. Khazaei and A. Amini-Manesh, Synthesis, 2005, 1929 CrossRef CAS.
- B. Maleki, M. Gholizadeh and Z. Sepehr, Bull. Korean Chem. Soc., 2011, 32, 1697 CrossRef CAS.
- S. Habibzadeh and H. Ghasemnejad-bosra, J. Chin. Chem. Soc., 2001, 58, 1 Search PubMed.
- S. F. Hojati, M. Gholizadeh, M. Haghdoust and F. Shafiezadeh, Bull. Korean Chem. Soc., 2010, 31, 3238 CrossRef CAS.
- General procedure for the synthesis of 1-carbamato-alkyl-2-naphthol derivatives: a mixture of 2-naphthols (0.288g, 2 mmol), aldehydes (2 mmol), benzylcarbamate (.320 g, 2.2 mmol) and (0.0464g TCCA or 0.0394g DCDMH, 0.2 mmol, 10 mol%), in a 10 mL round-bottomed flask sealed with a stopper, was stirred in an oil-bath (80 °C). After completion of the reaction, as monitored by TLC, The reaction was cooled to room temperature, washed with hot water and the pure solid products (compounds 1a–14a) were obtained by recrystallization from ethanol (95%). Benzyl (2-hydroxynaphthalen-1-yl)(3-nitrophenyl)methylcarbamate 3a: yellow solid; Rf (EtOAc/n-hexane: 4/6) = 0.50; m.p. 196–197 °C (ref. 26 mp 200–202 °C); 1H NMR (90 MHz, DMSO-d6): δ (ppm) 5.09 (2H), 6.94–8.12 (17H), 10.23 (s, 1H, OH); 13C NMR (22.5 MHz, DMSO-d6): δ (ppm) 50.26, 65.82, 117.80, 118.46, 120.50, 121.38, 122.60, 126.82, 127.57, 127.67, 128.24, 128.61, 129.57, 129.86, 131.91, 132.71, 144.99, 153.11, 156.28; IR (KBr, cm−1): 3389, 3352, 1694, 1629, 1527, 1514, 1438, 1348, 1333, 1276, 1233, 1142, 1048, 1016, 922, 830, 810, 743, 624. Benzyl(3-chlorophenyl) (2-hydroxynaphthalen -1-yl) methylcarbamate 6a: white solid; Rf (EtOAc/n-hexane: 4/6) = 0.65; m.p. 183–185 °C ; 1H NMR (90 MHz, DMSO-d6): δ (ppm) 5.09 (2H), 6.88-8.00 (17H), 10.19 (s, 1H, OH); 13C NMR (22.5 MHz, DMSO-d6): δ (ppm) 50.35, 65.82, 118.27, 118.52, 122.61, 124.77, 125.83, 126.34, 126.73, 127.55, 128.24, 128.45, 128.58, 129.59, 129.86, 131.99, 132.98, 136.89, 145.10, 153.00, 155.96; IR (KBr, cm−1): 3435, 3241, 1675, 1629, 1575, 1507, 1439, 1327, 1271, 1227, 1064, 947, 892, 813, 784, 754, 702. Benzyl (2,4-dichlorophenyl) (2-hydroxynaphthalen-1-yl) methylcarbamate 8a: white solid; Rf (EtOAc/n-hexane: 4/6) = 0.60; m.p. 202–204 °C (ref. 26 mp 208–210 °C); 1H NMR (90 MHz, DMSO-d6): δ (ppm) 5.05 (2H), 6.87–8.06 (16H), 9.94 (s, 1H, OH); 13C NMR (22.5 MHz, DMSO-d6): δ (ppm) 49.66, 65.53, 116.46, 118.66, 122.51, 126.50, 127.36, 127.59, 128.20, 128.56, 129.67, 131.19, 132.05, 132.55, 133.29, 137.06, 138.76, 153.59, 155.47; IR (KBr, cm−1): 3417, 3343, 3031, 1678, 1628, 1585, 1513, 1432, 1341, 1271, 1139, 1054, 936, 878, 844, 744.
- General procedure for the condensation between 2-naphthol, terephthaldehyde and alkyl carbamates: A mixture of 2-naphthols (0.288 g, 2 mmol), terephthaldehyde (0.268g, 2 mmol or 0.134g, 1 mmol), benzylcarbamate (0.320g, 2.2 mmol) and (0.0464g TCCA or 0.0394g DCDMH, 0.2 mmol, 10 mol%), in a 10 mL round-bottomed flask, was stirred in an oil-bath (80 °C). After completion of the reaction, as monitored by TLC, The reaction was cooled to room temperature, washed with hot water. Afterward, warm aqueous ethanol (15%, 30 mL) was added to crude products, and stirred for 10 min (1-carbamato-alkyl-2-naphthol 1b is soluble in warm aqueous ethanol and bis(1-amidoalkyl-2-naphthol) 2b is insoluble in this solvent). During this time, the crude 1-carbamato-alkyl-2-naphthol 1b was dissolved in the aqueous ethanol, and the pure bis(1-amidoalkyl-2-naphthol) 2b was remained. Thus, bis (1-amidoalkyl-2-naphthol) 2b was easily separated by filtration and pure 1-carbamato-alkyl-2-naphthol (1b) was recrystallized from EtOH(95%).
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra45036h |
| This journal is © The Royal Society of Chemistry 2014 |