Base stable quaternary ammonium ionic liquids†
Abstract
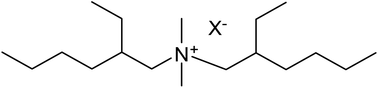
Ionic liquids with the bis(2-ethylhexyl)dimethylammonium cation, [BEDMA]+, were prepared by a halide-free route starting from the readily available secondary amine bis(2-ethylhexyl)amine. The following anions were considered: chloride, bromide, iodide, nitrate, hydrogensulphate, dihydrogenphosphate, formate, acetate, propionate, trifluoroacetate, methyl sulphate, methanesulphonate, tosylate, isonicotinate, nicotinate and picolinate. Several of the compounds are room-temperature ionic liquids, albeit with a high viscosity. All ionic liquids are soluble in water. The ionic liquids are very stable in strongly alkaline medium. No signs of decomposition could be observed by contact of the chloride ionic liquid with 50 wt% sodium hydroxide, even after prolonged heating at 80 °C. The high stability against strong bases is attributed to the branched structure of the quaternary ammonium cation, which blocks the Hofmann elimination reaction.

 Please wait while we load your content...
Please wait while we load your content...