Synthesis of thiodiazole copper microcapsules and release behavior of inhibiting R. solanacearum†
Chao Feng*,
Chengsheng Zhang,
Fanyu Kong and
Jing Wang
Tobacco Integrated Pest Management of China Tobacco, Tobacco Research Institute of Chinese Academy of Agricultural Sciences, Qingdao 266101, PR China. E-mail: fengchao020511@163.com; Fax: +86 532 88701012; Tel: +86 532 88703236
First published on 5th December 2013
Abstract
Microcapsules are one of the most useful devices to reduce the dosage of pesticides and prolong the duration of the active ingredient in target crops. In this study, thiadiazole copper (TDC) microcapsules were synthesized by in situ polymerization using poly(urea-formaldehyde) and characterized by field emission scanning electron microscopy, laser diffraction analysis, 3D optical microscopy, thermogravimetric analysis (TGA), and Fourier transform infrared spectroscopy. The effects of pH and temperature on the release of TDC were characterized by UV-visible absorption spectroscopy. The relationship between the micromechanical behavior of the microcapsules and release of TDC was studied by nanoindentation tests. The particle size distributions of the microcapsules (10–530 μm) were controlled by different reaction parameters. The microcapsules were stable below 220 °C, as determined by TGA. The release kinetics indicated that the higher the temperature, the faster the release rate. The TDC microcapsules were sensitive to pH, and the fastest release rate was observed at pH 4.0. The maximum load, hardness, and Young's modulus under the same displacement conditions decreased during the release. Water swelling was the major reason for TDC release from the microcapsules. The pot experiments confirmed that the microcapsules exhibited long-term sustained release of TDC, thereby protecting the tobacco from R. solanacearum.
Introduction
Pesticide chemicals play an important role in the control of pests and weeds diseases in agriculture and forestry; however, environmental pollution, residual toxicity, and human diseases are also caused by pesticides. Moreover, chemical manufacturing involves large consumption of energy and generates hazardous chemical waste.1,2 Therefore, reduced dosage of pesticides and prolonged duration of active ingredients in target crops are of significant interest.One of the effective ways to solve these problems is to control the release of active ingredients from polymeric microcapsules,3 which is of significant interest in drug delivery,4,5–8 fragrance release,9–11 food preservation,12 and self-healing materials.13,14 The utility of polymeric microcapsules for efficient cargo storage stems from their ability to deliver chemicals (e.g., pesticides) “just in time” to control the pests.15 Polymeric microcapsules protect the active ingredients from light and other environmental factors. Moreover, polymeric microcapsules effectively reduce chemical exposure, providing safety for the operators. Therefore, polymeric microcapsules meet some of the key requirements of green chemistry to the environment.16
Tobacco bacterial wilt, caused by a soil-borne pathogen R. solanacearum, is one of the most serious diseases affecting worldwide tobacco production.17 Moreover, thiodiazole copper (TDC), a new fungicide effective for living bacterial disease,18 is widely used in China. TDC is safe for crops, fishes, birds, honeybee, silkworms, human beings, animals, and beneficial insects, and does not cause environmental pollution. Therefore, TDC could be microencapsuled by polymers for control release applications.
Moreover, nanoindentation is a suitable direct method to obtain micromechanical properties of polymeric microcapsules, such as maximum load, hardness, and Young's modulus, compared to the indirect method (osmotic pressure method).19 The Oliver–Pharr method20 has been widely used for the measurement of elastic modulus and hardness via nanoindentation in characterizing the mechanical behavior of materials at small-scale.21–24 Lee et al.25 introduced the nano-indentation test to characterize the micromechanical behavior of poly(melamine-formaldehyde) (PMF), as a polymeric shell material. The PMF of self-healing microcapsules was shown to behave as a viscoelastic plastic material. Furthermore, Wang et al.26 introduced oxygen plasma treated carbon nanotubes to poly(urea-formaldehyde) (PUF) microcapsules to enhance the elasticity of the microcapsule shells during the polymerization. The improvement in micromechanical behavior of the microcapsule shells was confirmed by nanoindentation tests.
To the best of our knowledge, the synthesis of TDC microcapsules using PUF through in situ polymerization and the micromechanical behavior of microcapsule shells in the release process have not yet been reported. The objective of this research project was to synthesize TDC microcapsules that could minimize the initial release of the TDC, and instead provide a slow and sustained release to protect the tobacco from R. solanacearum. TDC was prepared via in situ polymerization, and the effects of pH and temperature on the release kinetics were studied. The changes in the micromechanical behavior of TDC microcapsules was analyzed by nanoindentation tests. The trends in the maximum load, hardness, and Young's modulus in the release process were discussed. The disease index and control effect of different pesticide treatments to protect the tobacco from R. solanacearum were studied by the pot experiments.
Materials and methods
Materials
TDC and 20% TDC suspension concentrate (TDC SC) were obtained from Zhejiang Longwan Chemicals Co., Ltd. Soybean oil fatty acid methyl esters (SOAME) were obtained from West Asia Chemical Corporation.Tobacco seeds of Honghua Dajinyuan variety, which was susceptible to tobacco bacterial wilt disease, were obtained from Tobacco Research Institute of Chinese Academy of Agricultural Sciences.
The water used in all the experiments was produced by a Millipore Milli-Q Plus 185 purification system and has a resistivity level higher than 18.1 MΩ cm. Hydrochloric acid, soda lye, and sodium sulfide nonahydrate were obtained from Beijing Chemical Works, CP. Sodium dodecyl benzene sulfonate, urea, 37 wt% formaldehyde solution, ammonium chloride, resorcinol, rhodamine B, and 1-octanol were obtained from Sigma Aldrich. Rhodamine B was used to probe the shell. Fluorol Green Golden 084 as a fluorescence probe for the core was obtained from Kunshan Haite Plastic Pigment Co., Ltd. All chemicals were used as received.
Characterization
The morphology, physical properties, and stability of polymeric microcapsules were characterized prior to embedding in the soil for crop protection. The shell wall integrity and the morphology of the microcapsules were observed by field emission scanning electron microscopy (FESEM) and laser diffraction analysis (LDA). The FESEM images of the polymeric microcapsules were acquired using a Hitachi S-4800 FESEM microscope. The optical microscopy images of the polymeric microcapsules were recorded using a VHX-1000, Keyence Co., Ltd.The core–shell structure was obtained by a confocal laser scanning microscope, Fluo ViewTM FV1000, Olympus Corp. Fluorol Green Golden 084 was excited using a diode-pumped solid-state laser with a wavelength of 488 nm; rhodamine B was excited using a helium-neon laser with a wavelength of 543 nm.
The TGA data of the polymeric microcapsules were examined using a TGA Q5000, TA Instrument.
The size distributions of microcapsules were obtained using a Mastersizer 2000 Version 5.54, Malvern Instruments Ltd.
Fourier transform infrared spectroscopy (FT-IR) tests were performed using a Thermo Scientific Nicolet iS10, Thermo Fisher Scientific Inc.
Nanoindentation tests were performed using an Agilent G200 NanoIndenter (Agilent Technologies, Inc., Chandler, AZ) with a three-sided pyramid diamond Berkovich indenter. The indenter has a normal angle of 65.3° between the tip axis and the faces of the triangular pyramid.
The release characterization of TDC microcapsules were examined by UV-visible transmittance spectra using a UV-3600 Shimadzu.
Microcapsule preparation
The microcapsules containing TDC were synthesized by in situ polymerization of urea and formaldehyde using a modified literature process,27,28 as shown in Fig. 1. TDC was completely dissolved in SOAME, and then the mixture (15.0% w/v) was slowly added to 35 mL of 1.5% sodium dodecyl benzenesulfonate, urea, resorcinol, and ammonium chloride at room temperature, and stirred for 30 min. One drop of ultrahydrophobic agent, either octane or hexadecane, was added to the TDC mixture, as a costabilizer to decrease Ostwald ripening28 and increase the hydrophobicity of the inner phase. Then, formalin (37% formaldehyde) was added in the same ratio as described previously by Wang et al.26 And, the pH was adjusted to 3.50 with sodium hydroxide. The temperature of bath was slowly raised to 60 °C at a rate of 1 °C min−1. After 4 h of continuous agitation, the heating and mechanical agitation were stopped. Then, the reaction mixture with the polymerized microcapsules were repeatedly washed with ethanol and water to remove the impurities.Determination of core content of microcapsule
The core content of TDC microcapsule was determined by extracting method with acetone as extracting solvent.29 The TDC microcapsules were grinded, collected and washed with acetone several times, then dried at room temperature. Knowing the initial weight of microcapsules (Mi), the weight of residual wall shell of microcapsules (Mr), the wall shell content (Ws) and core content (Wc) of microcapsules were calculated as
 | (1) |
| Wc = 1 − Ws | (2) |
Greenhouse pot experiments
The pot experiments were conducted using a modified process by Liu et al.30 in a greenhouse in Qingdao, Shan Dong province. The surface sterilization were used to treat the tobacco seeds with household bleach (2% NaOCl) for 6 min. Then, the seeds were rinsed six times in Milli-Q water, and sown in a nursery mixture of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 of vermiculite, perlite, and turf.31 The resulting medium was sterilized at 121 °C for 2 h and placed into a sterilized plastic tray. The plastic tray was placed over distilled water and kept at 28–30 °C and 60% relative humidity for approximately 60 days. Appropriate fertilizers were applied during the entire seedling stage.
4 of vermiculite, perlite, and turf.31 The resulting medium was sterilized at 121 °C for 2 h and placed into a sterilized plastic tray. The plastic tray was placed over distilled water and kept at 28–30 °C and 60% relative humidity for approximately 60 days. Appropriate fertilizers were applied during the entire seedling stage.
Infested soil inoculated by R. solanacearum
R. solanacearum was inoculated in a lysogeny broth medium and shaken on a rotary shaker for 24 h at 30 °C and 150 rpm. The bacterial pellets were suspended in sterilized water and mixed thoroughly with soils obtained from a field located in Fujian province. The soil properties are as follows: pH 6.50, 14.11 g kg−1 of total C, 2.02 g kg−1 of total N, 1.24 g kg−1 of total P, 12.35 mg kg−1 of available P, 10% of sand, 50% of silt, and 40% of clay. The concentration of R. solanacearum was adjusted to 10 cfu g−1 dry soil.32Results and discussion
Microcapsule size
The sizes of the microcapsules were analyzed by LDA. The sizes of the microcapsules were controlled by the agitation rate in the polymerization process.33The FE-SEM images of the TDC microcapsules under 500, 1000, and 1400 rpm agitation rates are shown in Fig. 2A, C and E, respectively.
 | ||
| Fig. 2 FE-SEM images and size distributions of microcapsules under different agitation rates. (A) and (B) under 500 rpm; (C) and (D) under 1000 rpm; (E) and (F) under 1400 rpm. | ||
The TDC microcapsules obtained under 500 rpm agitation rate had a diameter ranging from 85–530 μm, with a mean diameter of 283 μm (Fig. 2B). In the polymerization, shear forces generated by agitation could tear large oil droplets to small ones. The equilibrium between interfacial tension of the discrete oil droplets and shear forces depended on the agitation rate.34 Interfacial tension dominated at low agitation rate, and dispersed droplets remained large.
As the agitation rate was increased from 500 to 1000 rpm, the diameter of the microcapsules decreased to 50–400 μm, with a mean diameter of 130 μm (Fig. 2D). Further, as the agitation rate was increased from 1000 to 1400 rpm, the diameter of the microcapsules decreased to 15–105 μm containing well formed shell walls, and with a mean diameter of 44 μm (Fig. 2F). As strong shear forces were generated under high agitation rate, large droplets were broken up into small ones.35 Thus, the increase in the agitation rate resulted in smaller-diameter microcapsules.
The core contents of TDC microcapsules were 85.1, 78.7, and 65.4 under 500 rpm, 1000 rpm, and 1400 rpm, respectively. The core contents of microcapsules prepared at lower agitation rate were higher than that of microcapsules prepared at higher agitation rate.29 The weight fraction of core material in larger microcapsule was relatively higher. Whereas that in smaller microcapsule was relatively smaller.
Confocal fluorescence micrograph of TDC microcapsule was shown in Fig. 3. The core and the shell were labeled with Fluorol Green Golden 084 (green) and rhodamine B (red), respectively. In the fluorescence intensity cross-section profile (Fig. 3B), the intensity of the rhodamine B (red) had two side peaks that marked the shell. And, the intensity of the Fluorol Green Golden 084 (green) concentrated in the middle of the yellow line that marked the core. Thus, confocal fluorescence micrograph and the fluorescence intensity distribution demonstrated that the microcapsules had a core–shell structure.
 | ||
| Fig. 3 (A) Confocal fluorescence micrograph of TDC microcapsule. (B) Fluorescence intensity profile along the yellow line across the microcapsule in (A). | ||
Thermal stability of microcapsules
The thermal stability and overall quality of the TDC microcapsule were evaluated by TGA. The samples were examined using a TGA Q5000 apparatus under a nitrogen flow (30 mL min−1) at a heating rate of 10 °C min−1 from 25 to 550 °C. The samples (4–10 mg) were placed in a platinum crucible.Fig. 4 shows a comparison of TGA curves between the TDC microcapsules (with a stable shell wall) and TDC. All samples lost mass in a single step. The onset points of mass loss of the TDC, SOAME, TDC microcapsules, and microcapsule shells were 228, 165, 165, and 210 °C, respectively. The SOAME and TDC microcapsules showed a similar trend in mass loss under the same heating rate. The microcapsules were stable below 164 °C (Fig. 4b). The TGA of the microcapsules, prepared by the optimized process (1400 rpm agitation rate) showed <5% weight loss before 100 °C (residual water). Then, a sharp weight loss occurred between 160 and 240 °C, corresponding to TDC thermolysis (Fig. 4a) and loss of SOAME (boiling point 165 °C) (Fig. 4d). Thus, the microcapsules were stable untill the release of vaporized TDC. The TDC microcapsules were as stable as the PUF microcapsules containing epoxy resins, as reported by Cosco et al.36 The mass fraction of the TDC microcapsules and microcapsule shells were 10.6% and 7.9%, respectively, calculated by Wang et al.26 The residue consisted of the reacted shell materials. This phenomenon indicates that the TDC from the microcapsules were completely lost in the heating process below 321 °C.
 | ||
| Fig. 4 Curves for mass loss of TDC (a), TDC microcapsules (b), microcapsule shells (c), and SOAME (d) over the temperature range of 25 to 550 °C. | ||
FT-IR spectra
The FT-IR spectra of TDC, SOAME, microcapsules, and microcapsule shells are shown in Fig. 5. The strong transmittance peak at 1507.10 cm−1 in the spectrum for TDC is attributed to cyano (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) groups. The peak at 1604.93 cm−1 may be attributed to S–C
N) groups. The peak at 1604.93 cm−1 may be attributed to S–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups (overlapped with NH2 peak) (Fig. 5a). Further, carboxylate (COO−, 1455.79 cm−1) and carbonyl (C
N groups (overlapped with NH2 peak) (Fig. 5a). Further, carboxylate (COO−, 1455.79 cm−1) and carbonyl (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O, 1746.39 cm−1) peaks for SOAME were observed (Fig. 5b). These characteristic peaks were also observed in the FT-IR spectra of microcapsules, indicating the presence of TDC as the core material in the synthesized microcapsules (Fig. 5c). Furthermore, peaks for urea group (CO–NH) at 1628.12 cm−1 (overlapped with C
O, 1746.39 cm−1) peaks for SOAME were observed (Fig. 5b). These characteristic peaks were also observed in the FT-IR spectra of microcapsules, indicating the presence of TDC as the core material in the synthesized microcapsules (Fig. 5c). Furthermore, peaks for urea group (CO–NH) at 1628.12 cm−1 (overlapped with C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O peak), amine group (N–H) at 1560.82 cm−1, and cyano group (C–N) at 1434 cm−1 for the shells29 were observed (Fig. 5d). The characteristic peaks of shells are also present in the microcapsules, as shown in Fig. 5c. Thus, the comparison of FT-IR spectra confirmed that the synthesized microcapsules contain TDC as the core material.
O peak), amine group (N–H) at 1560.82 cm−1, and cyano group (C–N) at 1434 cm−1 for the shells29 were observed (Fig. 5d). The characteristic peaks of shells are also present in the microcapsules, as shown in Fig. 5c. Thus, the comparison of FT-IR spectra confirmed that the synthesized microcapsules contain TDC as the core material.
Characterization of TDC-release kinetics
Most of the prepared TDC microcapsules are circular in shape and without any cracks on the surface (Fig. 6A). During the immersion in soil, outer mechanical force could break the shells of microcapsules, thereby forming microcracks on their surfaces (Fig. 6B) and release the TDC from their core. | ||
| Fig. 6 Optical microscopy images of TDC microcapsules (A) and microcapsules with cracks on the surface of shell after outer mechanical force treatment (B), recorded by depth-from-defocus method. | ||
Because environmental factors such as temperature (20–40 °C) and soil acidity (pH 4.0–7.0) would affect the TDC microcapsules in the actual soil application trials, these factors were simulated for the release kinetics test.
The presence of TDC could not be directly analyzed by UV-visible absorption spectra. Therefore, it was indirectly analyzed by adding 0.1 M sodium sulfide nonahydrate (Na2S·9H2O) to TDC, producing UV-visible active tizanidine (AMT). The UV-visible absorption spectra of released AMT was recorded between 270 and 350 nm using a UV-3600 Shimadzu with a slit width of 1.0 nm, and 0.1 M Na2S·9H2O was used as the reference material.
The reaction between TDC and Na2S·9H2O producing AMT is shown in Fig. 7. AMT was used to indirectly measure the concentrations of released TDC.
 | ||
| Fig. 7 Reaction between TDC and Na2S·9H2O forming tizanidine (AMT), which is detected by UV-visible absorption spectra. | ||
The TDC microcapsules are light green in colour, as shown in Fig. 8A, while, the hydrolysate copper sulfide (CuS), produced from the reaction between TDC and Na2S·9H2O, is black in colour. Because the rough surfaces of the microcapsules adsorbed the CuS, they became gray in colour, as shown in Fig. 8B.
 | ||
| Fig. 8 Optical microscopy images of prepared microcapsules (A), and microcapsules released after treatment with 0.1 M Na2S·9H2O for 3 days (B). | ||
UV-visible spectra is a useful technique for determining the absorption wavelength of the UV-active products.37,38 The released concentrations of AMT at different immersion times were obtained from its UV-visible spectra. The increasing trend of the absorption peak at 290.2 nm indicates that TDC continually releases into the water (Fig. S1†).
The common semiempirical exponential equation were used to analyze the data obtained from TDC release experiments, and to evaluate the influence of pH and temperature on the release kinetics as follows:3,12
 | (3) |
The concentration gradient of a released active ingredient is the major driving force for a Fickian diffusion system whose diffusion parameter is close to 0.50. While, the erosion of particles is the dominant driving force for a degradation controlled-release system whose diffusion parameter is close to 1.0. In the case of spheres, n value should be corrected to 0.43 and 0.85 corresponding to Fickian diffusion and degradation controlled-release systems, respectively.40 As evidenced in Table 1, the TDC release from the microcapsules was controlled by Fickian diffusion.
| Sample | K | n | C | R | t50 (h) |
|---|---|---|---|---|---|
| P1 | 0.131 | 0.353 | 0.210 | 0.989 | 9.578 |
| P2 | 0.452 | 0.173 | −0.106 | 0.986 | 5.494 |
| P3 | 0.473 | 0.168 | −0.093 | 0.987 | 4.403 |
| P4 | 0.199 | 0.272 | 0.139 | 0.992 | 8.870 |
| T1 | 0.080 | 0.462 | 0.182 | 0.983 | 19.562 |
| T2 | 0.036 | 0.553 | 0.376 | 0.970 | 9.362 |
| T3 | 0.540 | 0.143 | −0.195 | 0.989 | 5.782 |
The correlation coefficient (R) for all formulations is >0.97, and indicates an excellent correlation of TDC release profile from the microcapsules, as shown by using eqn (3).
Using the data obtained for AMT, the corresponding TDC release profiles at various pH values and temperatures are shown in Fig. 9A and B, respectively. The TDC release concentrations at different pH values show the same trend. The release concentrations increase rapidly until 90 h. After that, the release concentrations increase slowly, and reaches a steady state concentration. However, the t50 values of different pH samples in Table 1 are 9.578, 5.494, 4.403, and 8.870 h for pH 4, 5, 6, and 7, respectively, which are all below 10 h. The t50 values at pH 4 and 7 are larger than those of pH 5 and 6, indicating that the microcapsules at pH 4 and 7 show long-term sustained release of TDC. Thus, the TDC microcapsules are sensitive to pH, as indicated by the release kinetic tests. However, the change in pH did not significantly change the value of n.
The shell made from PUF has a network structure at molecular level. Thus, water could penetrate into the shell and swell the shell of the microcapsule in the solution.19,41 On different pH conditions, crosslinking reaction occurred on the shell of the microcapsule. However, the effects of the changes in the pH on the shell has not been clearly interpreted. For TDC microcapsules, water swelling was the major reason for active ingredient release. The pH value influenced the crosslinking reaction of the shell, and had synergistic effect with water swelling.
In the temperature effect test, the t50 value for sample T1 is significantly larger than T2 and T3, indicating that the microcapsules at lower temperature show long-term sustained release of TDC. The t50 value changed from 19.562 to 5.782 h as the temperature was raised from 20 to 40 °C. Higher temperature could accelerate the rate of Fickian diffusion process, resulting in higher release rate. Therefore, as expected, temperature is a key parameter affecting the release process. The laboratory tests were conducted in water, and it is expected that the release rate would be much slower in the actual soil environment.
Nanoindentation test
The microcapsules need to be separated from one another for nanoindentation test. Thus, the microcapsules were dispersed in ethanol, and carefully separated on a smooth surface of glass slide. Then, the back of the glass slide was strongly adhered to a stainless steel sheet by hot melt adhesive. Moreover, the continuous stiffness measurement (CSM) mode was choosen for nanoindentation test at a constant rate of 0.05 s−1 up to 3 μm depth. Tests were performed at 25 °C. The holding time of indentation was 10 s. The curve was measured three times with the same sample on the distinct surface regions at each indentation load. The hardness and Young's modulus values were averaged over three indents. The microcapsule sizes are in the range 15–105 μm with a mean diameter of ∼55 μm (Fig. 8A).In the CSM technique, cycles of indentation, each of which consists of continuous incremental loading and partial unloading, are carried out until a final desired depth is obtained.42 Fig. 10A shows the typical loading–unloading curve of the TDC microcapsules at different immersion times. This behavior is similar to that of viscoelastic-plastic polymer43 and polymer films,44 respectively.
The “maximum load” could characterize the ability of microcapsules to endure outer stress.45 The maximum load of TDC microcapsules during the holding time was 29.192 mN after 30 min immersion, which is the largest load in the release process. This result indicates that the micromechanical behavior of the microcapsules in the initial immersion period is nearly similar to that of prepared microcapsules.
Then, in the continuous immersion release process, the maximum load of the microcapsules were 21.883, 9.734, 8.291, and 4.829 mN, corresponding to 34, 90, 228, and 480 h immersion, respectively. This finding proves that the immersion process weakens the microcapsule's ability to endure outer stress. These characteristics may correspond to the nature of the rough surface of the microcapsule shell, which become more viscoelastic after absorbing the water. Thus, the shell of microcapsules became more easily damaged under the influence of outer stress. The cracks increased in size and number on the shell surfaces, release more core materials (TDC).
Hardness is defined as a measurement of the material's resistance to local plastic deformation. Thus, the hardness, H, was calculated from the data from the loading curve and was defined as the maximum load, Pmax, divided by the contact indentation area, A.
 | (4) |
Young's modulus (E) was calculated from the following equation:
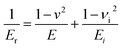 | (5) |
Young's modulus-displacement and hardness-displacement curves of the TDC microcapsules tested by using CSM at different immersion times are shown in Fig. 10B and C. Both the Young's modulus and hardness in the stable process show a decreasing trend in the release process, similar to the maximum load curves (Fig. 10A).
In the CSM test, the indenter became stable in the displacement from 2000 to 3000 nm. The entire data for Young's modulus and hardness are averaged in Fig. 10D. It shows a clear decreasing trend vs. immersion time.
The hardness and Young's modulus are 0.048 and 1.468 GPa after 30 min immersion, respectively. Both of them sharply decreased to 0.016 and 0.606 GPa after 4 h immersion, respectively. Further, they continuously decreased to 8.273 × 10−4 and 0.266 GPa after 480 h immersion, respectively.
As the microcapsules swelling in water, the network structure shell became loose and weak. The change of the physical structure led to the change of the micromechanical properties. Therefore, the maximum load, hardness, and Young's modulus exhibit similar decreasing trend during the TDC release process. All of them decreased sharply until 90 h immersion, then gradually decreased after 90 h. This trend is similar to the release kinetics of pH and temperature, as shown in Fig. 9. Thus, the micromechanical behavior of the TDC microcapsules was significantly influenced by immersion in the release process.
Pot experiment
TDC molecule has two active groups owning fungicidal effect (Fig. 7). One is thiazole group, which does not inhibit bacterial growth outside of plants. However, it is highly effective inside the plant. The active ingredient of TDC microcapsules taken up by the plant causes severe damage to bacteria by thinning the skin of bacteria leading to their death.49 The other is copper ion that strongly inhibit both fungal and bacterial growth. After exchanging with positive ions such as H+ and K+ ions on the cell surface of disease-producing germs, copper ions forms complex with the proteins of cell membrane leading to their death. Moreover, copper ions can also penetrate into the cells of disease-producing germs and form complex with enzymes, resulting in energy maladjustment leading to their death. Under the combined action of these two active groups, TDC can provide perfect fungicidal effect on different germs.In the pot experiment, the concentration of R. solanacearum was adjusted to approximately 10 cfu g−1 soil. The soils inoculated without (for A treatment) and with (for B–D treatments) pathogens were defined as health control and disease control, respectively. The bacterial counts of R. solanacearum in the various treatments are listed in Tables 2, which were determined at 3 days, 10 days, and 20 days after the transplantation. Four treatments in total were carried out. Each treatment had three blocks (three replicates) which were randomly laid out, and each block for each treatment had four pots. Thus, each treatment had 12 pots. One tobacco seedling was transplanted into each pot.
| Treatments | Details | 3 days | 10 days | 20 days | |||
|---|---|---|---|---|---|---|---|
| Disease index | Control effect (%) | Disease index | Control effect (%) | Disease index | Control effect (%) | ||
| A | Soils without inoculation of R. solanacearum | — | — | — | — | — | — |
| B | Soils inoculated with R. solanacearum | 1.8 | — | 12.7 | — | 30.1 | — |
| C | Soils treated with TDC SC and then inoculated with R. solanacearum | 0 | 100 | 4.4 | 65.4 | 12.7 | 57.8 |
| D | Soils treated with TDC microcapsules and then inoculated with R. solanacearum | 0 | 100 | 2.0 | 84.2 | 7.5 | 75.1 |
After about 60 days of incubation in nursery beds, the tobacco seedlings were transplanted into soils. For C (TDC SC) treatment, the tobacco seedlings were directly transplanted into the soil inoculated with 10 mL (0.05 g mL−1 active ingredient [ai]). For D (the TDC microcapsules were synthesized inhouse) treatment, the tobacco plants were transplanted into the soils that had been mixed with 0.5 g of ai.
The four treatment samples did grow well for 3 days, as shown in Fig. 11. However, one leaf of sample B became wilted, indicating that the tobacco was infected with the inoculated R. solanacearum after 3 days. Further, sample B has did not inhibit the propagation of R. solanacearum. The disease index of sample B is 1.8, while the disease index and control effect of both samples C and D are 0 and 100%, as listed in Table 2.
Moreover, some yellow areas were observed on the wilted leaf of sample B, indicating that R. solanacearum could continuously infect the plant until 10 days.
Meanwhile, no wilted leaves were observed in samples A and D for 10 days. However, one leaf of sample C became wilted in the same time, indicating that TDC SC could not effectively control R. solanacearum, with a control effect of 65.4%.
At 20 days after the transplantation, samples B and D became completely wilted. The soils treated with pesticides (C and D) had smaller counts of R. solanacearum than the control soil. The disease incidence for samples C and D decreased to 57.8% and 75.1%, respectively, with the application of TDC. Moreover, the disease indexes for sample B, C, and D declined from 30.1 to 12.7 and 7.5 after 20 days.
In the pot experiments, water, existing in the soil, could penetrate into the shells and swell the shells of the microcapsules. Due to the shell swelled, micro channels formed in the shells, and the release process occurred.
Because of the slow and controlled release of TDC from the microcapsules, the treated tobacco plants could be protected for a long time. Therefore, the control effect of sample D is better than that of sample C. It confirms that the microcapsules effectively control the release of TDC in the pot experiment to protect the tobacco from R. solanacearum.
Thus, the application of TDC microcapsules significantly suppressed the bacterial infection compared to TDC SC, both at 10 and 20 days after the transplantation. This effectiveness was increased when the pesticide was applied with the rain water.
Conclusions
TDC microcapsules were synthesized via in situ polymerization. The size distributions of the microcapsules depend on the agitation rates during the polymerization. The FT-IR spectra of the microcapsules and precursors confirmed that TDC was successfully encapsulated. The microcapsules were stable under 220 °C, as measured by TGA. The release concentration of TDC during the release process was measured by UV-visible absorption spectra. The release concentration of TDC significantly increased until 90 h immersion, and then gradually maintained a steady state concentration. For PUF microcapsules, water swelling was the major reason for TDC release. The release kinetics demonstrated that the TDC microcapsules are sensitive to pH, and the highest release rate was observed at pH 6. Further, higher temperature accelerated the release rate of TDC microcapsules. The maximum load, hardness, and Young's modulus under the same displacement conditions were decreased in the release process. The pot experiments demonstrated that the TDC microcapsules were very effective against R. solanacearum in vitro in plant owing to slow and sustained long-term release of the active ingredient (TDC).Acknowledgements
This work was financially supported by National Natural Science Foundation of China (no. 31000878). The authors thank Dr Wei Wang from Luoyang Ship Material Research Institute for his support of nanoindentation measurements.Notes and references
- N. Benmouhoub, N. Simmonet, N. Agoudjil and T. Coradin, Green Chem., 2008, 10, 957–964 RSC.
- X. Wang and J. Zhao, J. Agric. Food Chem., 2013, 61, 3789–3796 CrossRef CAS PubMed.
- P. Stloukal, P. Kucharczyk, V. Sedlarik, P. Bazant and M. Koutny, J. Agric. Food Chem., 2012, 60, 4111–4119 CrossRef CAS PubMed.
- S. Zhang, Z. Chu, C. Yin, C. Zhang, G. Lin and Q. Li, J. Am. Chem. Soc., 2013, 135, 5709–5716 CrossRef CAS PubMed.
- L. Zhou, M. Chen, L. Tian, Y. Guan and Y. Zhang, ACS Appl. Mater. Interfaces, 2013, 5, 3541–3548 CAS.
- K. E. Broaders, S. J. Pastine, S. Grandhe and J. M. J. Frechet, Chem. Commun., 2011, 47, 665–667 RSC.
- S. De Koker, R. Hoogenboom and B. G. De Geest, Chem. Soc. Rev., 2012, 41, 2867–2884 RSC.
- G. Verma and P. A. Hassan, Phys. Chem. Chem. Phys., 2013, 15, 17016–17028 RSC.
- A. C. Balazs, Mater. Today, 2007, 10, 18–23 CrossRef CAS.
- M. Andersson Trojer, L. Nordstierna, M. Nordin, M. Nyden and K. Holmberg, Phys. Chem. Chem. Phys., 2013, 15, 17727–17741 RSC.
- T. Dispinar, C. A. L. Colard and F. E. Du Prez, Polym. Chem., 2013, 4, 763–772 RSC.
- J. Asrar, Y. Ding, R. E. La Monica and L. C. Ness, J. Agric. Food Chem., 2004, 52, 4814–4820 CrossRef CAS PubMed.
- A. P. Esser-Kahn, S. A. Odom, N. R. Sottos, S. R. White and J. S. Moore, Macromolecules, 2011, 44, 5539–5553 CrossRef CAS.
- S. A. Odom, A. C. Jackson, A. M. Prokup, S. Chayanupatkul, N. R. Sottos, S. R. White and J. S. Moore, ACS Appl. Mater. Interfaces, 2011, 3, 4547–4551 CAS.
- I. M. Martins, S. N. Rodrigues, M. F. Barreiro and A. E. Rodrigues, Ind. Eng. Chem. Res., 2012, 51, 11565–11571 CrossRef CAS.
- M. A. Albrecht, C. W. Evans and C. L. Raston, Green Chem., 2006, 8, 417–432 RSC.
- P. Tang, X. Xiong and J. Du, Agric. Sci. Technol. Equip., 2008, 3, 35–36 Search PubMed.
- L. Zhang, J. Wang, G.-N. Zhu and L. Su, Exp. Toxicol. Pathol., 2010, 62, 163–169 CrossRef CAS PubMed.
- C. Gao, S. Leporatti, S. Moya, E. Donath and H. Möhwald, Langmuir, 2001, 17, 3491–3495 CrossRef CAS.
- W. C. Oliver and G. M. Pharr, J. Mater. Res., 2011, 7, 1564–1583 CrossRef.
- C. A. Schuh, Mater. Today, 2006, 9, 32–40 CrossRef CAS.
- W. C. Oliver and G. M. Pharr, J. Mater. Res., 2011, 19, 3–20 CrossRef.
- H. M. Song, Y. J. Kim and J. H. Park, J. Phys. Chem. C, 2008, 112, 5397–5404 CAS.
- D. Sun, Q. Fu, Z. Ren, H. Li, D. Ma and S. Yan, Polym. Chem., 2014, 5, 220–226 RSC.
- J. Lee, M. Zhang, D. Bhattacharyya, Y. C. Yuan, K. Jayaraman and Y. W. Mai, Mater. Lett., 2012, 76, 62–65 CrossRef CAS PubMed.
- W. Wang, L. Xu, F. Liu, X. Li and L. Xing, J. Mater. Chem. A, 2013, 1, 776–782 CAS.
- E. N. Brown, M. R. Kessler, N. R. Sottos and S. R. White, J. Microencapsulation, 2003, 20, 719–730 CAS.
- B. J. Blaiszik, N. R. Sottos and S. R. White, Compos. Sci. Technol., 2008, 68, 978–986 CrossRef CAS PubMed.
- L. Yuan, G. Liang, J. Xie, L. Li and J. Guo, Polymer, 2006, 47, 5338–5349 CrossRef CAS PubMed.
- Y. Liu, J. Shi, Y. Feng, X. Yang, X. Li and Q. Shen, Biol. Fertil. Soils, 2012, 49, 447–464 CrossRef.
- X. Y. Dai, Y. R. Su, W. X. Wei, J. S. Wu and Y. K. Fan, J. Exp. Bot., 2008, 60, 279–289 CrossRef PubMed.
- F. Lemessa and W. Zeller, Biol. Control, 2007, 42, 336–344 CrossRef PubMed.
- L. Yuan, G.-Z. Liang, J.-Q. Xie, J. Guo and L. Li, Polym. Degrad. Stab., 2006, 91, 2300–2306 CrossRef CAS PubMed.
- J. M. Rallison, Annu. Rev. Fluid Mech., 1984, 16, 45–66 CrossRef.
- J. D. Rule, N. R Sottos and S. R. White, Polymer, 2007, 48, 3520–3529 CrossRef CAS PubMed.
- S. Cosco, V. Ambrogi, P. Musto and C. Carfagna, J. Appl. Polym. Sci., 2007, 105, 1400–1411 CrossRef CAS.
- P. Papaphilippou, M. Christodoulou, O.-M. Marinica, A. Taculescu, L. Vekas, K. Chrissafis and T. Krasia-Christoforou, ACS Appl. Mater. Interfaces, 2012, 4, 2139–2147 CAS.
- K. Vasilev, N. Poulter, P. Martinek and H. J. Griesser, ACS Appl. Mater. Interfaces, 2011, 3, 4831–4836 CAS.
- S. Zuleger and B. C. Lippold, Int. J. Pharm., 2001, 217, 139–152 CrossRef CAS.
- J. Siepmann and N. A. Peppas, Adv. Drug Delivery Rev., 2012, 64, 163–174 CrossRef PubMed.
- H. Masoud and A. Alexeev, ACS Nano, 2012, 6, 212–219 CrossRef CAS PubMed.
- X. Li and B. Bhushan, Mater. Charact., 2002, 48, 11–36 CrossRef CAS.
- J. Menčík, L. H. He and J. Němeček, Polym. Test., 2011, 30, 101–109 CrossRef PubMed.
- T.-H. Fang and W.-J. Chang, Microelectron. J., 2004, 35, 595–599 CrossRef CAS PubMed.
- D. M. Ebenstein and L. A. Pruitt, Nano Today, 2006, 1, 26–33 CrossRef.
- W. C. Oliver and G. M. Pharr, MRS Bull., 2011, 35, 897–907 CrossRef.
- S. Varughese, M. S. R. N. Kiran, K. A. Solanko, A. D. Bond, U. Ramamurty and G. R. Desiraju, Chem. Sci., 2011, 2, 2236–2242 RSC.
- L. Feng, M. Chittenden, J. Schirer, M. Dickinson and I. Jasiuk, J. Biomech., 2012, 45, 1775–1782 CrossRef PubMed.
- H. Yang, W. Zhang, Q. Kong, H. Liu, R. Sun, B. Lin, H. Zhang and Z. Xi, Food Chem. Toxicol., 2013, 53, 100–104 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional UV-visible absorption spectra and grade investigating method of tobacco disease are provided. See DOI: 10.1039/c3ra45744c |
| This journal is © The Royal Society of Chemistry 2014 |