Self-assembly of discotic liquid crystal decorated ZnO nanoparticles for efficient hybrid solar cells†
Abstract
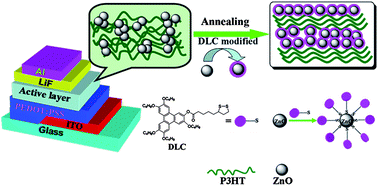
Efficient hybrid solar cells based on a blend of poly(3-hexylthiophene) (P3HT) and discotic liquid crystal ligands dithiol-functionalized triphenylene (TP-S) modified zinc oxide (ZnO) nanoparticles (TP-S@ZnO) were systematically investigated. The TP-S-modified ZnO nanoparticles possess a well-defined dispersibility, especially after annealing under the liquid-crystalline state (130 °C), originating from the help of the supramolecular self-assembly of the TP-S discs. Discotic liquid crystal ligands improve the compatibility between P3HT polymer and ZnO nanoparticles, which is beneficial for enhanced charge separation and transfer efficiency. On the other hand, the interfacial molecules TP-S can play a great role in the ordering and crystallinity of P3HT chains. X-ray diffraction (XRD) and wide-angle X-ray scattering (WAXS) studies indicate that the spontaneous self-assembly of the promotes P3HT chains to overall, the power conversion efficiency (PCE) of polymer/ZnO hybrid solar cells increased from 0.46% to 0.95% after ZnO was modified with TP-S under thermal annealing. As expected, DLC molecular interface modification can provide a viable and interesting method to promote the compatibility and a large interfacial area between polymers and nanocrystals, subsequently improving the performance of photovoltaic devices.

 Please wait while we load your content...
Please wait while we load your content...