Design and fabrication of random silver films as substrate for SERS based nano-stress sensing of proteins†
Jayakumar Perumal‡
b,
Kien Voon Kong‡b,
U. S. Dinishb,
Reuben M. Bakker*a and
Malini Olivo*bc
aAdvanced Concepts and Nanotechnology Division, Data Storage Institute, Agency for Science Technology and Research, DSI building, 5, Engineering Drive 1, Singapore 117608. E-mail: Reuben_BAKKER@dsi.a-star.edu.sg
bBio-Optical Imaging Group, Singapore Bioimaging Consortium, Agency for Science Technology and Research (A*STAR), 11 Biopolis Way, #02-02 Helios, Singapore 138667. E-mail: Malini_Olivo@sbic.a-star.edu.sg
cSchool of Physics, National University Ireland, University Road, Galway, Ireland
First published on 23rd January 2014
Abstract
We report a simple and easy to fabricate random silver film (RSF) as a highly sensitive Surface Enhanced Raman Scattering (SERS) substrate which can be fabricated directly onto a dielectric substrate such as glass. An electron beam evaporation system was used for substrate fabrication. The SERS activity is attributed to the formation of electromagnetic ‘hot-spots’ on the film. Substrate performance is analyzed by studying the reproducibility and signal enhancement from the Raman active molecule, 2-naphthalene thiol (NT), which is covalently anchored to the substrate. The metal thickness is optimized to achieve the highest SERS enhancement. Based on this study we found that a 7 nm RSF substrate gave the best SERS activity. The SERS signal intensity exhibited by 7 nm RSF is found to be at least 3 orders of magnitude higher than that of a commercial substrate. The SERS enhancement factor is estimated to be ∼1 × 107 with a point-to-point intensity variation of about 12% and it reaches a maximum of 15% for batch-to-batch comparison. The efficacy of this substrate for biosensing is demonstrated by detecting H1 influenza protein, and the detection limit is found to be ∼10 pM when it is used along with a recently established nano-stress SERS sensor, 4-ATP (4-amino-thiophenol), as linker molecule. This detection limit shows a performance superior to conventional ELISA (which has a nM detection limit). These results show promise for the development of a biosensing platform based on the marriage of RSF with nano-stress sensors.
1. Introduction
Surface-Enhanced Raman Scattering (SERS) techniques have progressed dramatically from the originally observed enhancement on roughened silver electrodes to the current fields of sensing and imaging, single molecule detection and other applications.1 This is achieved when the analyte molecules are physically or chemically adsorbed on to the substrate. SERS substrates are available with variety of structural morphologies, including roughened metal electrodes,2–4 aggregated films,5 metal islands of different morphology,6–9 metals film over nanospheres (MFON),10 porous films,11 bimimitic substrates,12 nanogap structured films,13 and semi-continuous films.14–16 While these substrates have been successfully applied in many important sensing applications,17–20 these substrates either need long processing times or high fabrication costs and in addition they do not fulfil the requirements for all SERS sensing applications. The fabrication of plasmonic substrates with high reproducibility and high sensitivity towards SERS based biosensing is required.Herein, we have proposed a process of preparing the plasmon active substrate, which will be mass-producible with a large pattern area following a simple fabrication process. Hence we have proposed the fabrication of a random silver film (RSF). This fabrication was achieved using an E-beam evaporator; either glass or another dielectric substrate can be used as the base substrate material. The RSF concept has been studied for several decades. The optical properties of these metal–dielectric composites are governed by surface plasmon resonance (SPR), which is the collective oscillation of conduction electrons in a metal structure under incident light. Plasmonic nanostructures act like optical nanoantennae, accumulating and building up electromagnetic energy on the nanometer-scale in so-called ‘hot spots’.21,22 This results in high local fields and thus can produce a tremendous enhancement of the optical response. RSF on dielectric substrates typically grow according to the Volmer–Weber growth mode,23 which results in three dimensional islands on the substrate surface.
Biosensing methods with SERS generally fall into one of two categories: direct sensing and indirect sensing. The direct detection method is based on the distinct vibration information of target bio-molecule itself, whereas in the indirect detection method, one detects the target bio-molecule by observing a known Raman probe (also called a Raman tag) that indicates the presence of the molecule one is searching for.19,24,25 Despite this, the direct detection strategy is a better way for reliable and convenient detection for any analyte, compared to the indirect method of detection. This is because direct sensing is limited by the low intrinsic Raman cross section of biomolecules. This in turn leads to poor reproducibility arising from biological targets with high molecular weight, such as proteins.26 It is very difficult to carry out highly sensitive measurements. In indirect sensing, the biomolecule binding event is confirmed by the presence of a Raman label27–30 which requires molecular recognition events that have been made possible by labeling either the capture agent or the target molecule itself with a Raman active molecule. Another way of conducting labelling is by attaching the Raman active molecule onto the metal nanoparticles. This structure is then used to capture and detect the biomolecule of interest through their specific binding sites.31–33
The most recent advancement in this direction is a novel form of the “label free detection” method, which does not involve the use of a secondary antibody. Briefly, the linker or anchoring molecule for antibody immobilisation also functions as a Raman tag. In which any binding event on the Raman tag is detected by small shifts in the tag's Raman spectrum. This results from the binding of the biomolecule of interest onto an antibody-conjugated Raman active molecule.34 The observed frequency shifts were attributed to structural deformations in the antibody-conjugated SERS reporter molecule as a result of the antibody–antigen binding event. The working mechanism of this technique can be described as a nano-mechanical biosensor. This combination provides a novel biosensing technique with high selectivity and possibility for label-free biomolecule detection.
Hence, in our current work we report the use of RSF as a high enhancement factor SERS substrate to detect H1 influenza protein as protein of interest by using 4-ATP as a stress sensor, to determine the limit of detection of this biosensor.
2. Experimental
2.1. Materials used
Chemicals were obtained from Aldrich and used as received. Acetone (99%), IPA (99%), DI water, and Ag deposition materials were obtained from Alfa Aesar. 4-Aminothiophenol, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS), glycine, ethanol and PBS were purchased from Sigma-Aldrich. H1 antibody and antigen were purchased from Abcam.2.2. SERS substrates
Random Silver Films (RSFs) were fabricated using electron beam evaporation on borosilicate glass. Prior to deposition, the substrates were cleaned with acetone, isopropanol alcohol and DI water and then dried on a hotplate. A BOC Edwards (EB3) AUTO 306 E-beam evaporator was used in this experiment. The evaporator was pumped down to 10−6 to 10−7 millibar. The deposition rate (kept at 0.05 nm s−1) and film thicknesses were monitored with a quartz crystal oscillator. Films of various thicknesses were grown using different deposition times. A field emission scanning electron microscope (FE-SEM) from Joel was used to image the samples.2.3. Commercial substrate
For comparison, we used a commercial SERS substrate: Klarite™ from D3 technologies. Klarite is a 6 mm × 10 mm silicon substrate coated with Au and an active nanostructured area of size 4 mm × 4 mm.2.4. NT SERS measurements
Substrates were tested for SERS activity with naphthalenethiol (NT), a Raman active molecule.35 This experiment was conducted purely to identify and select the exact mass average thickness of RSF with the highest SERS activity from the different thicknesses of RSF substrates under study. NT cannot be used for the protein binding study as they lack a reactive site for antibody binding. NT (10 μM solution in ethanol) was incubated on the substrate for 2 hours and then washed with ethanol to remove unbound NT. The intensity of the 1066 cm−1 Raman peak was used for comparison.13,36,37 To evaluate reproducibility, spectra were measured from ten random locations (1 mm apart) on each substrate. The relative standard deviation (RSD) of the intensity value is also calculated.2.5. SERS nano-stress sensor
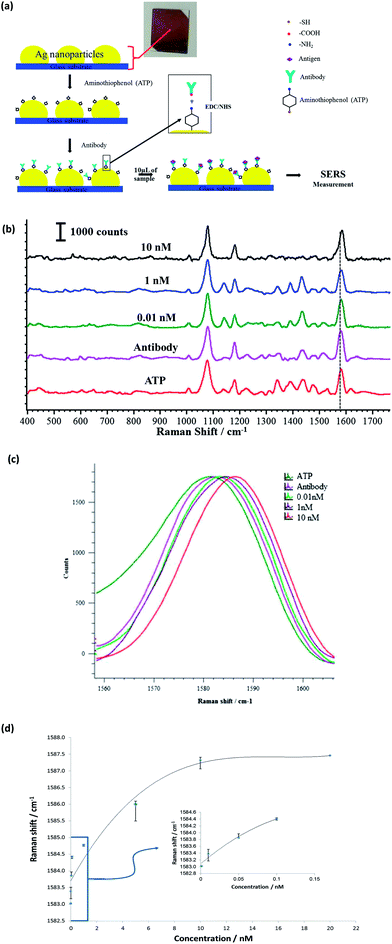
For the nano-stress sensor, we used 4-aminothiophenol (4-ATP) as SERS active reporter. This linker acts as a transducer and demonstrates a Raman frequency shift due to nano-mechanical stress. SERS substrates were cleaned with ethanol and dried in an argon gas environment. The substrates were immersed in a 10 mM 4-ATP solution prepared in ethanol for 1 h. The substrates were washed with ethanol to remove unbound 4-ATP, followed by rinsing with PBS. As a result the RSF substrate was coated with a monolayer of ATP, as depicted in Fig. 3a. SERS spectra of the immobilized 4-ATP molecule were measured using a Raman microscope. The antibody solution (5 μL) was mixed with 50 μL of EDC (150 mM) and 50 μL NHS (400 mM) solution and the resulting mixture was allowed to react for 5 min. For anti-H1 antibody conjugation, the 4-ATP coated substrates were incubated in the mixture for 2 h at room temperature. The conjugated substrates were removed from the mixture and washed with PBS.This anti-H1/ATP coated substrate was blocked by 0.1 mM glycine to improve the binding specificity. H1 protein detection was started by incubating different concentrations of pure H1 protein solution as shown in Fig. 3b. The incubation time was maintained at 30 minutes.
2.6. Raman measurements
Raman measurements were performed in reflection mode with a Raman microscope (Renishaw InVia) using a 633 nm excitation laser, a 1800 line per mm grating and a cooled CCD (−70 °C). A 50× objective lens (NA 0.75) delivered the laser beam and collected the back scattered light. Rayleigh scattering was blocked with a notch filter. The laser spot size was ∼1 μm with a power of 0.3 mW. Measurements were performed with a 10 second integration time. Measurements were taken at multiple positions across each SERS region and data was averaged. Background corrections and curve fittings were carried out using WiRE 3.2 (Renishaw software). Spectra were background subtracted by a 6-order polynomial fit before the curve-fitting procedure. The instrument is calibrated with signal from a silicon standard at 520 cm−1.3. Result & discussion
3.1. Characterization of SERS substrates
Random silver films of mass averaged thickness from 3 nm to 20 nm were fabricated as described above. This technique results in Volmer–Weber growth. Small isolated metal granules are first formed on the base substrate. As the silver coverage increases through further deposition, the granules coalesce on to the substrate surface. The deposition process is stopped before a continuous silver layer is formed, resulting in various sizes of silver particles and their aggregates. The results of this are viewed in Fig. 1 which shows FE-SEM images of substrates with various mass average thickness of RSF. The images show metal nanoparticles and their aggregates in grey and dielectric material (the substrate) in dark grey or black. | ||
| Fig. 1 SEM image showing the surface morphology of different thickness of RSF substrates, scale bar is 100 nm. | ||
For low thicknesses, small, isolated, spheroidal metal particles are formed on the substrate. Spheroidal metal particles are found for 3–7 nm thick films (the average particle size was measured as: 280 nm2 for 3 nm thick films, 435 nm2 for 5 nm thick films and 775 nm2 for 7 nm thick films). As the thickness of RSF increases, the particles coalesce into islands of irregular shapes (average size of 7290 nm2 for 9 nm thick films). As the thickness increases even more, a continuous path is formed through the silver island; this point is called the percolation threshold. Beyond this, the coverage fraction increases until the metal completely covers the sample surface; this occurs at approximately 20 nm for RSF. See ESI for more details.†
The samples were studied for optimal SERS activity. A Raman active molecule, naphthalenethiol (NT), was chosen to assess the enhancement performance of the RSF substrate. Substrates were prepared for measurement as mentioned above. A representative Raman spectrum of NT is illustrated in Fig. 2a. To compare the SERS performance of different substrates, the SERS intensity at the 1066 cm−1 peak was calculated at normalized experimental settings.13,36,37 Results from the SERS performance of RSF substrates with different mass average thickness are shown in Fig. 2b. The Raman peak intensity for NT (excited at 633 nm) increased from the 3 nm sample to the 5 nm sample and to the 7 nm sample. Starting with the 9 nm sample, the Raman signal decreased. Among the substrates tested, the 7 nm RSF sample shows the highest SERS intensity but with an RSD of about 12% point-to-point variation and 15% for batch-to-batch variation.
Experiments were repeated for the 7 nm substrate from different batches; the results were consistent. The RSD of the signal intensity from other RSF substrates was in the range of 20–60%. We observed that 7 nm RSF substrates always showed better reproducibility compared to other RSF substrates. In case of the commercial substrate (Klarite™), RSD is found to be in the range of 7–9%, which is in good agreement with previously reported data.13 The signal enhancement from the commercial substrate was weak and it is at least 3 orders of magnitude lower than the 7 nm RSF substrate.
The SERS enhancement factor, G is studied for the 7 nm RSF substrate. It is calculated by comparing the Raman signal of NT with the 7 nm RSF substrate to that of a thick liquid layer of NT. G is expressed as
 | (1) |
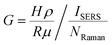 | (2) |
3.2. Bio-analysis – detection of H1 antigen using RSF substrate
Detection of the H1 antigen was performed using the novel combination of the 7 nm RSF and SERS-active linkers as a nano-mechanical stress sensor. In this technique, the vibrational frequencies of antibody conjugated SERS-active reporter molecules on a SERS substrates are shifted in quantitative correlation with the concentration of the targeted antigen. This frequency shift is attributed to mechanical deformation in the stress sensor due to antibody–antigen interaction forces. However, this technique was only able to achieve detection sensitivity (2 nM) comparable to that of a conventional sandwich immunoassay.34 In order to improve the detection limit of this technique, we used novel RSF as SERS substrate which can give a strong and reproducible signal. The SERS response of anti-H1 conjugated 4-aminothiophenol (4-ATP; linker) on 7 nm RSF was investigated.Herein, Fig. 3a shows the schematic for the working mechanism of protein sensing using a stress sensor concept. The SERS spectra of the anti-H1/ATP as a function of H1 protein concentrations varying from 0.01 nM to 10 nM is shown in Fig. 3b. As can be seen in the Figure, the peak at 1580 cm−1 corresponding to benzene ring stretching is particularly responsive to antibody–antigen binding, where 1580 cm−1 is up-shifted due to deformation of the benzene ring, as is clearly shown in high magnification image in Fig. 3c. Fig. 3d shows the dose–response curve for the H1 protein, and the detection limit of this SERS active linker based assay was found to be 0.01 nM which is more sensitive than the reported nano-mechanical sensor and conventional sandwich immunoassays.34,39,40 In our experimental setup we reached the highest detection point with 10 nM of H1 antigen. Beyond this it shows no peak shift, confirming the saturation level, since all the active sites have been occupied by the proteins already. This detection range is very useful when there is a trace level of antigen concentration in the bio-fluid. For detection of higher antigen concentration, we can rely on more conventional ELISA techniques.
Stability of the substrate is crucial to realize successful SERS-based sensing. We have examined the shelf-life of the 7 nm RSF substrate. To achieve this, we first exposed these substrates to PBS solution for a period of five consecutive days as shown in the ESI.† The substrate was then treated with ATP and the SERS intensity was measured to grade its stability. There was no significant degradation observed from the 7 nm RSF substrate, showing a reduction in SERS intensities to nearly 5%. Notably, the result substantiates the potential of the 7 nm RSF substrate for use in long-term sensing. Thus, the increased stability is likely to allow extended chemical treatment for functionalizing the surface, particularly useful in biosensing applications.
In this study, we have shown that the combination of the RSF substrate and SERS-active linker as a nano-mechanical stress sensor could generate a novel biosensor with high selectivity for biomolecules. It is possible to have high-density biomolecules sensing on single substrate, since the required sensing area is about 1 μm (Fig. 3). We propose that properly designed stress-sensitive and SERS active reporters could lead to a novel way to detect binding events in sub-micrometre bio-features.
4. Conclusion
We have successfully demonstrated a simple and straightforward method to fabricate SERS active RSF with a mass-average thickness of 7 nm, which is below the percolation threshold, and it can be used as an efficient substrate for biosensing; particularly in combination with the nano-stress sensor concept. These SERS substrates demonstrated at least 3 orders of magnitude higher signal enhancement than commercial substrates. Detection of H1 influenza protein with a limit of detection of ∼10 pM was achieved using 7 nm RSF substrate in combination with the nano-stress SERS sensor design. This sensitivity is beyond conventional ELISA (which has a detection limit in the nM range). These results show a significant advancement towards the development of a point-of-care SERS-based biosensor. Currently we are investigating the application of the RSF substrate for the development of various SERS based biosensor platforms.Acknowledgements
The authors acknowledge support from Science and Engineering Research Council (SERC) grant (no. 102 152 0011) of Agency for Science, Technology and Research (A*STAR).Notes and references
- B. Sharma, R. R. Frontiera, A.-I. Henry, E. Ringe and R. P. Van Duyne, Mater. Today, 2012, 15, 16–25 CrossRef CAS.
- M. Fleischmann, P. J. Hendra and A. J. McQuillan, Chem. Phys. Lett., 1974, 26, 163–166 CrossRef CAS.
- D. J. Jeanmaire and R. P. Van Duyne, J. Electroanal. Chem., 1977, 84, 1–20 CrossRef CAS.
- M. G. Albrecht and J. A. Creighton, J. Am. Chem. Soc., 1977, 99, 5215–5217 CrossRef CAS.
- C. Y. Chen, E. Burstein and S. Lundquist, Solid State Commun., 1979, 32, 63–66 CrossRef CAS.
- C. Y. Chen and E. Burstein, Phys. Rev. Lett., 1980, 45, 1287–1291 CrossRef CAS.
- J. G. Bergman, D. S. Chemla, P. F. Liao, A. M. Glass, A. Pinczuk, R. M. Hart and D. H. Olson, Opt. Lett., 1981, 6, 33–35 CrossRef CAS.
- D. A. Weitz, S. Garoff and T. J. Gramila, Opt. Lett., 1982, 7, 168–170 CrossRef CAS.
- P. K. Chang and T. E. Furtak, in Surface Enhanced Raman Scattering, Plenum Press, New York, 1982, p. 361 Search PubMed.
- L. A. Dick, A. D. McFarland, C. L. Haynes and R. P. Van Duyne, J. Phys. Chem. B, 2002, 106, 853–860 CrossRef CAS.
- L. H. Qian, X. Q. Yan, T. Fujita, A. Inoue and M. W. Chen, Appl. Phys. Lett., 2007, 90, 153120–153122 CrossRef PubMed.
- N. L. Garrett, P. Vukusic, F. Ogrin, E. Sirotkin, C. P. Winlove and J. Moger, J. Biophotonics, 2009, 2, 157–166 CrossRef CAS PubMed.
- U. S. Dinish, F. C. Yaw, A. Agarwal and M. Olivo, Biosens. Bioelectron., 2011, 26, 1987–1992 CrossRef CAS PubMed.
- P. Gadenne, D. Gagnot and M. Masson, Phys. A, 1997, 241, 161–165 CrossRef CAS.
- A. K. Sarychev and V. M. Shalaev, Phys. Rep., 2000, 335, 275–371 CrossRef CAS.
- V. M. Shalaev, Nonlinear Optics of Random Media: Fractal Composites and Metal-Dielectric Films, Springer Tracts in Modern Physics, Springer, Heidelberg, 2000, vol. 158 Search PubMed.
- R. M. Jarvis and R. Goodacre, Anal. Chem., 2004, 76, 40–47 CrossRef CAS PubMed.
- W. R. Premasiri, D. T. Moir, M. S. Klempner, N. Krieger, G. Jones and L. D. Ziegler, J. Phys. Chem. B, 2005, 109, 312–320 CrossRef CAS PubMed.
- Y. M. C. Cao, R. C. Jin and C. A. Mirkin, Science, 2002, 297, 1536–1540 CrossRef CAS PubMed.
- K. Kneipp, Y. Wang, R. R. Dasari, M. S. Feld, B. D. Gilbert, J. Janni and J. I. Steinfeld, Spectrochim. Acta, Part A, 1995, 51, 2171–2175 CrossRef.
- V. M. Shalaev, R. Botet and A. V. Butenko, Phys. Rev. B: Condens. Matter, 1993, 48, 6662–6664 CrossRef CAS.
- A. K. Sarychev, V. A. Shubin and V. M. Shalaev, Phys. Rev. B: Condens. Matter, 1999, 60, 16389–16409 CrossRef CAS.
- M. Ohring, The Materials Science of Thin Films, Academic Press, San Diego, 1992 Search PubMed.
- W. E. Doering and S. M. Nie, Anal. Chem., 2003, 75, 6171–6176 CrossRef CAS PubMed.
- Y. C. Cao, R. C. Jin, J. M. Nam, C. S. Thaxton and C. A. Mirkin, J. Am. Chem. Soc., 2003, 125, 14676–14677 CrossRef CAS PubMed.
- X. X. Han, B. Zhao and Y. Ozaki, TrAC, Trends Anal. Chem., 2012, 38, 67–78 CrossRef CAS PubMed.
- K. Hering, D. Cialla, K. Ackermann, T. Dörfer, R. Möller, H. Schneidewind, R. Mattheis, W. Fritzsche, P. Rösch and J. Popp, Anal. Bioanal. Chem., 2007, 390, 113–124 CrossRef PubMed.
- M. D. Porter, R. J. Lipert, L. M. Siperko, G. Wang and R. Narayanana, Chem. Soc. Rev., 2008, 37, 1001–1011 RSC.
- H. Cho, B. R. Baker, S. Wachsmann-Hogiu, C. V. Pagba, T. A. Laurence, S. M. Lane, L. P. Lee and J. B. H. Tok, Nano Lett., 2008, 8, 4386–4390 CrossRef CAS PubMed.
- X. X. Han, L. J. Cai, J. Guo, C. X. Wang, W. D. Ruan, W. Y. Han, W. Q. Xu, B. Zhao and Y. Ozaki, Anal. Chem., 2008, 80, 3020–3024 CrossRef CAS PubMed.
- M. Schutz, D. Steinigeweg, M. Salehi, K. Kompe and S. Schlucker, Chem. Commun., 2011, 47, 4216–4218 RSC.
- N. Guarrotxena, B. Liu, L. Fabris and G. C. Bazan, Adv. Mater., 2010, 22, 4954–4958 CrossRef CAS PubMed.
- M. Y. Sha, H. X. Xu, M. J. Natan and R. Cromer, J. Am. Chem. Soc., 2008, 130, 17214–17215 CrossRef CAS PubMed.
- K. W. Kho, U. S. Dinish, A. Kumar and M. Olivo, ACS Nano, 2012, 6, 4892–4902 CrossRef CAS PubMed.
- P. Jiang, A. Nion, A. Marchenko, L. Piot and D. Fichou, J. Am. Chem. Soc., 2006, 128, 12390–12391 CrossRef CAS PubMed.
- C. Y. Fu, K. W. Kho, U. S. Dinish, Z. Y. Koh and M. Olivo, J. Raman Spectrosc., 2012, 43, 977–985 CrossRef CAS.
- K. M. Balss, T. C. Kuo and P. W. Bohn, J. Phys. Chem. B, 2003, 107, 994–1000 CrossRef CAS.
- R. R. Kolega and J. B. Schlenoff, Langmuir, 1998, 14, 5469–5478 CrossRef CAS.
- S. Vavassori, A. Kumar, G. S. Wan, G. S. Ramanjaneyulu, M. Cavallari, S. E. Daker, T. Beddoe, A. Theodossis, N. K. Williams, E. Gostick, D. A. Price, U. S. Dinish, K. V. Kong, M. Olivo, J. Rossjohn, L. Mori and G. D. Libero, Nat. Immunol., 2013, 14, 908–916 CrossRef CAS PubMed.
- L. Guerrini, E. Pazos, C. Penas, M. E. Vázquez, J. L. Mascareñas and R. A. Alvarez-Puebla, J. Am. Chem. Soc., 2013, 135, 10314–10317 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra44867c |
| ‡ Joint first authors who contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |