An ultrasensitive SERS method for the determination of ozone using a nanogold sol as substrate and rhodamine S as probe†
Xinghui Zhang,
Chenyin Lin,
Qingye Liu* and
Aihui Liang*
Key Laboratory of Ecology of Rare and Endangered Species and Environmental Protection, Ministry of Education, Guangxi Normal University, Guilin 541004, P. R. China. E-mail: zljiang@mailbox.gxnu.edu.cn; Fax: +86-0773-5846201; Tel: +86-0773-5846141
First published on 14th October 2013
Abstract
In an aggregated gold nanoparticle (AuNP) sol substrate, rhodamine S (RhS) exhibited a surface-enhanced Raman scattering (SERS) peak at 1503 cm−1. Based on the ozone oxidization and RhS–I3 particle reactions, 1.56–62.5 nmol L−1 of O3 can be determined by SERS, with a detection limit of 0.9 nmol L−1.
Ozone is a light blue gas that exists in the atmospheric layer 10–50 km from the ground, and strongly absorbs ultraviolet rays from the sun, especially UV-B, which is harmful to biological organisms. Since ozone can protect all life on earth from UV rays, it enables life on earth to exist, reproduce and develop. Ozone is also a strong oxidizer that can oxidize all metals except gold and platinum, and can destroy C–C bonds in organic compounds. In urban areas, NOx from vehicle exhaust emissions and volatile organic compounds from factory waste gases photochemically react in the sun to produce ozone. If there is little cloud and the wind is weak, the formed ozone accumulates and causes ozone pollution. China's air quality standards specify the maximum average concentration limit of 8 hours of ozone per day as 160 μg m−3 in the living environment. When the concentration of ozone exceeds the limit, it will cause environmental pollution which is harmful to human health.1 Thus, the rapid, accurate and sensitive detection of ozone is important.
At present, several methods have been reported for the determination of ozone, including spectrophotometry,2–4 fluorescence,5–8 chemiluminescence,9–11 high performance liquid chromatography-mass spectrometry (HPLC-MS),12 electrochemistry13 and resonance Rayleigh scattering.14 Among them, the sensitivity of the colorimetric method is low,3 and its measurement concentration range is from 0.003–2 mg m−3. Chemiluminescence has a high sensitivity, fast response, and good specificity, but its operation is complicated. HPLC-MS has a good selectivity, but the cost is too high. The electrochemical method was used in a reactor containing composite multilayered hydrophobic C-18 which absorbed ozone selectively,14 and its detection limit was 60 μmol L−1. Amos5 used 2-diphenylacetyl-1,3-indandione-1-hydrazone as a fluorescence reagent to determine ozone in concentrations as low as 20 ng mL−1. Xu et al. presented a near-infrared fluorescent probe of L-tryptophan-tricarbocyanine that was utilized to determine 0.025–7 μmol L−1 of ozone.7
As an optical analytical technique, surface-enhanced resonance Raman scattering (SERS) spectroscopy has been attracting much attention due to its unique properties including providing a spectrum with an enormous amount of informational content, narrow spectral bands, being free from photobleaching, and having a high sensitivity. At present, SERS has been widely applied in the detection of inorganic ions, small biomolecules, nucleic acid, proteins, bacteria, and cells.15–19 While significant advances have been made in engineering various SERS-active metal (mainly Ag and Au) nanostructures,20–25 SERS is far from being adopted as a routine analytical technique, especially in quantitative analysis.26–31 This is mainly due to the strong dependence of SERS enhancement on structural and morphological features of the metal nanostructure substrate as well as on the surface chemistry and molecular binding properties. Commonly used solid metal nanostructure substrates are simple and sensitive, but their reproducibility is not ideal. The nanogold sol is one of best substrates, combining low cost, convenience, simplicity, sensitivity and reproducibility. To the best of our knowledge, SERS methods for inorganic molecules such as Hg2+, Cd2+, As3+, Ni2+ and Cl− have been reported.25,32 However, a rapid, convenient and sensitive SERS method for the quantitative determination of ozone has not yet been reported, using a nanogold sol as the substrate. This article describes a new, sensitive and accurate SERS method for the determination of ozone in air samples, using RhS as a molecular probe on an aggregated AuNP sol substrate.
The selection of molecular probe is very important in SERS analysis and rhodamine dye is a very important and sensitive SERS molecular probe. Based on the reported references,15b rhodamine 6G (Rh6G) was commonly used as it was the most sensitive. In this work, rhodamine S (RhS) is the most sensitive dye for determining ozone and was selected for use from four rhodamine dyes which were found to not exhibit SERS effects in the absence of aggregated AuNPs. RhS exhibited a strong SERS effect in the aggregated AuNP solution system containing 0.18 mol L−1 HCl. O3 oxidized I− to produce I3−, which reacted with RhS to form a RhS–I3 associated particle which then aggregated into big particles by means of intermolecular forces, especially hydrophobic forces, during which the RhS molecules were enwrapped in the particle and this resulted in SERS quenching. The more ozone, the more I3− formed, and the more the SERS decreased due to more SERS RhS molecules being enwrapped in the body of the (RhS–I3)n associated particles. Based on this process, a highly sensitive SERS method was developed to determine the O3 concentration (Fig. 1). Similarly to SERS analysis, a simple fluorescence method can also be developed for the determination of trace ozone, based on oxidation and particle reactions, and without the addition of AuNPs. The main reactions are as follows, and the molar concentration of O3 is equal to the concentration of I2 and I3−. Thus, I3− can be used as the substitute standard solution for O3 which is unstable and not easy to obtain.
| O3 + 2I− + 2H+ = I2 + O2 + H2O | (1) |
| I− + I2 = I3− | (2) |
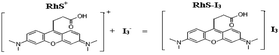 | (3) |
| n(RhS–I3) = (RhS–I3)n particle | (4) |
In the presence of 0.18 mol L−1 HCl and 21.52 mg L−1 AuNPs, RhS exhibited eight strong SERS peaks at 1120, 1176, 1195, 1273, 1304, 1355, 1503, and 1590 cm−1 (Fig. 2). With the addition of O3, the concentration of formed I3− increased, and the SERS intensity at 1503 cm−1 decreased greatly due to the formation of the (RhS–I3)n associated particles, in which the RhS molecules were enwrapped in the body of the particles. Thus, the SERS wavenumber of 1503 cm−1 was selected for use. The rhodamine 6G (Rh6G), rhodamine B (RhB) and butyl rhodamine B (b-RhB) systems were considered (see ESI, Fig. 1S–3S†), their most sensitive wavenumbers were 1503 cm−1, 1503 cm−1 and 1522 cm−1, respectively, and their sensitivities were inferior to the RhS system.
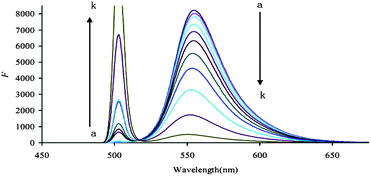
In 0.18 mol L−1 HCl and in the absence of the AuNPs, when the excited wavelength λex was 500 nm, RhS exhibited a strong fluorescence peak at 554 nm and a very weak Rayleigh scattering (RS) peak at 500 nm (Fig. 3). With the addition of O3, the concentration of the formed I3− increased, the fluorescence intensity at 556 nm decreased and the RS intensity increased accordingly due to the formation of the (RhS–I3)n associated particles, in which the RhS molecules were enwrapped. The enhanced RS peak indicated that there are particles in the system. Thus, a fluorescence wavelength of 554 nm was selected for use. The Rh6G, RhB and b-RhB systems were considered (see ESI, Fig. 4S–6S†) and their fluorescence wavelengths were 556 nm, 583 nm and 585 nm, respectively. The RhS system is the most sensitive of these fluorescence methods which are inferior to their corresponding SERS methods. However, the operation and accuracy of these fluorescence methods are better than the SERS methods.
The solid powder of KI3 was prepared by mixing and grinding equal molar amounts of KI (1.0 g) and I2 (1.5 g). The (RhS–I3)n powder was prepared by mixing and grinding 0.42 g of KI3 and 0.50 g of RhS. 0.20 g of the (RhS–I3)n powder was dissolved in 4 mL of ethanol and 1 mL of water, ground well and dried with IR rays to obtain the (RhS–I3)n associated particle sample. The IR spectra (see ESI, Fig. 7S†) showed that the peak at 3239 cm−1 is due to the stretching vibration of the O–H bond, the 2975 cm−1 peak is ascribed to the stretching vibration of the C–H bond in the methyl, the peak at 1716 cm−1 is due to the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, the four peaks at 1606 cm−1, 1528 cm−1, 1501 cm−1 and 1444 cm−1 belong to the stretching vibrations of the C
O bond, the four peaks at 1606 cm−1, 1528 cm−1, 1501 cm−1 and 1444 cm−1 belong to the stretching vibrations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in the benzene ring, the peak at 1646 cm−1 is ascribed to the stretching vibration of C
C bonds in the benzene ring, the peak at 1646 cm−1 is ascribed to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, the peak at 1313 cm−1 is due to the stretching vibration of the C–N bond, and the two peaks at 1248 cm−1 and 1181 cm−1 are ascribed to the stretching vibration of the C–O bond. After the formation of RhS–I3, the O–H stretching vibration peak changed from 3239 cm−1 to 3379 cm−1 due to an increase in the dipole moment of O–H caused by the association of the I3−, and the other peaks did not change. This showed that the compound is an associated complex.
N, the peak at 1313 cm−1 is due to the stretching vibration of the C–N bond, and the two peaks at 1248 cm−1 and 1181 cm−1 are ascribed to the stretching vibration of the C–O bond. After the formation of RhS–I3, the O–H stretching vibration peak changed from 3239 cm−1 to 3379 cm−1 due to an increase in the dipole moment of O–H caused by the association of the I3−, and the other peaks did not change. This showed that the compound is an associated complex.
The O3 oxidized the excess I− to form the anion I3− that combined with the cation RhS+ to produce the RhS–I3 associated complex through the ionic bond. There are hydrophobic interactions and intermolecular forces in these associated complexes and large (RhS–I3)n associated particles were formed. This was proved by laser scattering tests that indicated the sizes were distributed from 40 nm to 700 nm, and the average particle size was 490 nm (see ESI, Fig. 8S†), which is also in agreement with that of eqn 4.
In the SERS detection technique,33–36 solid substrates are commonly used. However, the reproducibility is not ideal, and there are difficulties for use in routine quantitative analysis although some high-cost reproducible solid substrates such as the tip of TERS and nano-arrays are reported. Some nanosols with good reproducibility and low-cost can be made using modern nanosynthesis, and their shape can also be controlled accurately. In this article, stable AuNP sols were prepared and considered as a SERS substrate.
The effect of RhS concentration on ΔI (= Iblank − Iozone) was tested. As shown in Fig. 9S,† when the concentration of RhS is 1.5 × 10−7 mol L−1, the system is at a maximum of ΔI. So, a 1.5 × 10−7 mol L−1 RhS concentration was chosen. The effect of HCl on ΔI was tested. In the absence of HCl, the SERS signal of RhS is zero due to Cl− causing the aggregation of AuNPs. When the concentration is 0.18 mol L−1, ΔI is the biggest (Fig. 10S†), and therefore this concentration was chosen for use. The effect of AuNP concentration was considered. In the absence of the AuNPs, the SERS signal of RhS is zero due to the lack of aggregated AuNPs. Fig. 11S† shows that ΔI increased with an increase in concentration of the AuNPs that were aggregated into clusters. The concentration of 21.52 mg L−1 AuNPs gave the biggest ΔI value, and was selected for use. The effect of coexistent substances on the determination of O3 was investigated, with a relative error of less than ±5%. The results showed (Table 1S†) that common substances such as metal ions or an oxidizer did not interfere with the determination of 20 nmol L−1 O3. This showed that the SERS method has good selectivity.
The analytical features of the eight SERS and fluorescence systems are listed in Table 2S.† The SERS analytical systems are more sensitive than the corresponding fluorescence systems by between ten and a hundred times. Among the SERS systems, the RhS system is the most sensitive and was chosen for use. Compared to the reported methods,2–14 this RhS SERS method is more sensitive, and it is one of the most sensitive methods for ozone at present. In addition, the reagent RhS is easy to obtain. Furthermore, the working curve of ΔI/Ib vs. ozone concentration is shown in Fig. 12S.† This new SERS method has been applied to the analysis of ozone in air samples and the results (Table 3S†) were in agreement with that of the indigo carmine spectrophotometer,2 with a relative standard deviation (RSD) of 2.0–3.7%.
In summary, ozone oxidizes excess I− to produce I3− which combines with RhS to form (RhS–I3)n associated particles, in which the RhS molecules are enwrapped in the particle body and cannot emit the SERS and fluorescence signals. Under the chosen conditions, the SERS quenching on the AuNP aggregates and fluorescence quenching are linear to O3 concentration. Thus, two types of highly sensitive and selective, simple and rapid SERS and fluorescence methods were developed to determine the O3 concentration in air samples. In addition, the chemical reaction and signal quenching mechanisms were also discussed.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21165005, 21267004, 21307017, 21367005), the Natural Science Foundation of Guangxi (no. 2013GXNSFFA019003) and Guangxi Key Laboratory of Environmental Pollution Control Theory and Technology.Notes and references
- GB 3095-2012, Air Quality Standard, Chinese Environmental Scientific Press, Beijing, 2012 Search PubMed.
- HJ 504-2009, Determination of O3 in air-indigo carmine spectrophotometry, Chinese Environmental Scientific Press, Beijing, 2009 Search PubMed.
- HJ 590-2010, Determination of O3 in air-UV spectrophotometry, Chinese Environmental Scientific Press, Beijing, 2010 Search PubMed.
- H. Tomiyasu, J. Am. Chem. Soc., 1984, 56, 752 CAS.
- D. Amos, Anal. Chem., 1970, 42, 842 CrossRef CAS.
- A. L. Garner, M. S. C. Claudette, R. P. Bruce, D. L. George, A. Shin and K. Kazunori, Nat. Chem., 2009, 1, 316 CrossRef CAS PubMed.
- K. Xu, S. X. Sun, J. Li, L. Li, M. M. Qiang and B. Tang, Chem. Commun., 2012, 48, 684 RSC.
- E. P. Felix, J. P. Filho, G. Garcia and A. A. Cardoso, Microchem. J., 2011, 99, 530 CrossRef CAS PubMed.
- M. Ermel, R. Oswald, J. C. Mayer, A. Moravek, G. Song, M. Beck, F. X. Meixner and I. Trebs, Environ. Sci. Technol., 2013, 47, 1930 CrossRef CAS PubMed.
- W. Qi, D. Wu, J. M. Zhao, Z. Y. Liu, W. Zhang, L. Zhang and G. B. Xu, Anal. Chem., 2013, 85, 3207 CrossRef CAS PubMed.
- C. Eipel, P. Jeroschewski and I. Steinke, Anal. Chim. Acta, 2003, 491, 145 CrossRef CAS.
- P. Wentworth Jr, J. Nieva, C. Takeuchi, R. Galve, A. D. Wentworth, R. B. Dilley, G. A. Delaria, A. Saven, B. M. Babior, K. D. Janda, A. Eschenmoser and R. A. Lerner, Science, 2003, 302, 1053 CrossRef PubMed.
- (a) Y. Ishii, T. A. Ivandini, K. Murata and Y. Einaga, Anal. Chem., 2013, 85, 4284 CrossRef CAS PubMed; (b) D. V. Stergiou, M. I. Prodromidis, P. G. Veltsistas and N. P. Evmiridis, Anal. Chem., 2006, 78, 4676 CrossRef CAS PubMed.
- C. Y. Lin, G. Q. Wen, A. H. Liang and Z. L. Jiang, RSC Adv., 2013, 3, 6627 RSC.
- (a) J. Hu and C. Y. Zhang, Anal. Chem., 2010, 82, 8991 CrossRef CAS PubMed; (b) Z. L. Jiang, D. M. Yao, G. Q. Wen, T. S. Li, B. Chen and A. H. Liang, Plasmonics, 2013, 8, 803 CrossRef CAS.
- Y. Z. Chu, M. G. Banaee and K. B. Crozier, ACS Nano, 2010, 4, 2804 CrossRef CAS PubMed.
- S. Huh, J. Park, Y. S. Kim, K. S. Kim, B. H. Hong and J. M. Nam, ACS Nano, 2011, 5, 9799 CrossRef CAS PubMed.
- (a) X. X. Han, B. Zhao and Y. Ozaki, TrAC, Trends Anal. Chem., 2012, 38, 67 CrossRef CAS PubMed; (b) S. J. Long, L. Li, H. Guo, W. Yang and F. Lu, Dyes Pigm., 2012, 95, 473 CrossRef CAS PubMed.
- S. Schluter, N. Popovska-Leipertz, T. Seeger and A. Leipertz, Phys. Procedia, 2012, 39, 835 CrossRef PubMed.
- Y. Han, R. Lupitskyy, T. M. Chou, C. M. Stafford, H. Du and S. Sukhishvili, Anal. Chem., 2011, 83, 5873 CrossRef CAS PubMed.
- G. Rusciano, A. C. D. Luca, G. Pesce, A. Sasso, G. Oliviero, J. Amato, N. Borbone, S. D'Errico, V. Piccialli, G. Piccialli and L. Mayol, Anal. Chem., 2011, 83, 6849 CrossRef CAS PubMed.
- R. A. Alvarez-Puebla and L. M. Liz-Marzan, Angew. Chem., Int. Ed., 2012, 51, 11214 CrossRef CAS PubMed.
- C. Lofrumento, M. Ricci, E. Platania, M. Becucci and E. Castellucci, J. Raman Spectrosc., 2013, 44, 47 CrossRef CAS.
- W. Xie, B. Walkenfort and S. Schlucker, J. Am. Chem. Soc., 2013, 135, 1657 CrossRef CAS PubMed.
- Y. Q. Wang, B. Yan and L. X. Chen, Chem. Rev., 2013, 113, 1391 CrossRef CAS PubMed.
- M. J. Banholzer, J. E. Millstone, L. Qin and C. A. Mirkin, Chem. Soc. Rev., 2008, 37, 885 RSC.
- J. Li, L. Chen, T. Lou and Y. Wang, ACS Appl. Mater. Interfaces, 2011, 3, 3936 CAS.
- S. Mabbott, E. Correa, D. P. Cowcher, J. W. Allwood and R. Goodacre, Anal. Chem., 2013, 85, 923 CrossRef CAS PubMed.
- D. Tsoutsi, J. M. Montenegro, F. Dommershausen, U. Koert, L. M. Liz-Marzan, W. J. Parak and R. A. Alvarez-Puebla, ACS Nano, 2011, 5, 7539 CrossRef CAS PubMed.
- S. Kasera, F. Biedermann, J. J. Baumberg, O. A. Scherman and S. Mahajan, Nano Lett., 2012, 12, 5924 CrossRef CAS PubMed.
- J. L. Duan, M. Yang, Y. C. Lai, J. P. Yuan and J. H. Zhan, Anal. Chim. Acta, 2012, 723, 88 CrossRef CAS PubMed.
- G. Q. Wen, A. H. Liang and Z. L. Jiang, Plasmonics, 2013, 8, 899 CrossRef CAS.
- B. Guo, Y. J. Tang, J. S. Luo and J. P. Cheng, Precious Met., 2008, 29(2), 5 CAS.
- Y. Q. Wang, B. Yan and L. X. Chen, Chem. Rev., 2013, 113, 1391 CrossRef CAS PubMed.
- I. Diez and R. H. A. Ras, Nanoscale, 2011, 3, 1963 RSC.
- Q. Zhang, C. H. Moran, X. H. Xia, M. Rycenga, N. X. Li and Y. N. Xia, Langmuir, 2012, 28, 9047 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra44668a |
| This journal is © The Royal Society of Chemistry 2014 |