Two-photon active polymeric nanoparticles for high contrast in vitro imaging†
Soumitra
Satapathi‡
ab,
Anoop K.
Pal‡
cd,
Lian
Li
e,
Lynne A.
Samuelson
e,
Dhimiter
Bello
d and
Jayant
Kumar
*ab
aDepartment of Physics and Applied Physics, University of Massachusetts Lowell, Lowell, MA 01854, USA. E-mail: Jayant_Kumar@uml.edu
bCentre for Advanced Materials, University of Massachusetts Lowell, Lowell, MA 01854, USA
cBiomedical Engineering and Biotechnology Program, University of Massachusetts Lowell, Lowell, MA 01854, USA
dDepartment of Work Environment, University of Massachusetts Lowell, Lowell, MA 01854, USA
eU.S. Army Natick Soldier Research, Development and Engineering Centre, Natick, MA 01760, USA
First published on 14th November 2013
Abstract
Two water dispersible and two-photon (TP) active polymer nanoparticles were synthesized from poly[2-(3-thienyl) ethanol n-butoxy carbonyl methyl-urethane] and poly(3,3′′′-didodecyl quarter thiophene) polymers using a simple miniemulsion technique. Strong TP fluorescence was observed from each of these nanoparticles using a femtosecond laser operated at 800 nm. Interestingly, these polymeric nanoparticles were found to be non-toxic in dose dependent cell viability studies for 24 h nanoparticle incubation time period. TP microscopic imaging and Z-stacking confirmed cellular internalization of TP active nanoparticles by human macrophage THP-1 cells. These fluorescent polymer nanoparticles could be suitable candidates for in vitro TP bioimaging.
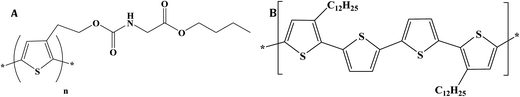
Over the past few years, numerous developments in fluorescence microscopy have pioneered the design of novel imaging modalities for high resolution cellular and tissue imaging.1–3 Considerable progress has been made in the area of two-photon fluorescence (TPF) microscopy due to its potential application in three-dimensional imaging of cancer cells,4 tissue engineering5 and immunology.6 TPF microscopy offers several advantages over the conventional confocal fluorescence microscopy, such as higher localization of fluorescence emission,7 reduction of background optical noise8 and improved spatial resolution.9 The low background fluorescence signals in TPF provides improved signal to noise ratio, leading to enhanced detection limits for sub-cellular structural imaging. Moreover, biological tissues are almost transparent in near-infrared (IR) wavelength region, where near-IR laser excitation is often used for two-photon (TP) imaging.10 The increasing interest in TPF microscopy has led to the design and synthesis of new TP biomarkers with higher TP-absorption (TPA) cross-sections.11–13 Colloidal quantum dots (QD) and gold nanorods are promising contrast agents for in vivo imaging and possess large TPA cross-sections. Although QDs have excellent photostability and narrow fluorescence spectral width, their application is limited by cytotoxicity, difficulty in surface modification and presence of dark dots in biological samples. Organic small molecules with the existence of symmetric charge transfer and large quadrupole moments have been reported to have large values of the TPA action cross-sections.14 Conjugated polymers are known to possess large optical nonlinearities, high absorption coefficients and high quantum efficiency of fluorescence.15 Recently, we have reported strong TPF from a soluble thiophene polymer, poly[2-(3-thienyl) ethanol n-butoxy carbonyl methyl-urethane] (PURET).16 Despite the excellent optical properties of conjugated polymers, their intrinsic hydrophobicity limits their application in bioimaging.18 Towards this end, aqueous conjugated polymer nanoparticles could be an excellent alternative.
Here, we report the fabrication, characterization and TP bioimaging properties of two water dispersible and fluorescent polymer nanoparticles of PURET17 and poly(3,3′′′-didodecyl quarter thiophene) (ADS12PQT) (acquired from American Dye Source, Inc). The chemical structures of the two thiophene polymers used for fabrication of nanoparticles are shown in Fig. 1. These nanoparticles were fabricated with surfactant assisted miniemulsion technique using an anionic surfactant sodium dodecyl sulphate (SDS). These fluorescent polymer nanoparticles are TP active. These nanoparticles didn't affect the cell morphology and cell viability of THP-1 cells and were readily internalized by macrophages as confirmed by TP confocal imaging.
Polythiophenes are known to have large third-order nonlinearities due to the π electron delocalization in their backbone and were chosen for nanoparticles fabrication. The two polythiophenes used are very soluble in organic solvents and possess large value of quantum efficiency of fluorescence. During the miniemulsion process, the chloroform droplets were formed in the presence of the aqueous surfactant solution. High energy homogenization was required to obtain a narrow particle size distribution. The probe sonication was adapted for achieving smaller droplets sizes. Initially, the nanoparticle size increased with the amount of mechanical agitation. The nanoparticle size then changed rapidly throughout the sonication process to approach a pseudo-steady state. Once this state was reached, particle size did not change. Then homogenization began, leading to the very broad size distribution of the nanoparticle. As constant fusion and fission processes proceeded, the polydispersity decreased and the miniemulsion reached a steady state. In the continuous phase, the droplets' surfaces were not completely covered with the surfactant molecules. Finally, the evaporation of chloroform removed the trapped solvent, resulting in the stable nanoparticles in the aqueous dispersion. The co-surfactant 1-pentanol provided the surfactant monolayer rigidity. The nanoparticles dispersions obtained were quite stable under ambient condition.
The fabricated nanoparticles were characterized by UV-vis absorption and fluorescence spectroscopies. Fig. 2(A) and (B) show the absorption and fluorescence spectra of the PURET and ADS12PQT nanoparticles, respectively. The PURET nanoparticles show an absorption maximum around 440 nm with a tail extending up to 600 nm. The 20 nm bathochromic shifts in the absorption spectrum in comparison to that for the PURET polymer solution confirmed the nanoparticles formation. The similar trend was observed for the ADS12PQT nanoparticles. The ADS12PQT nanoparticles exhibited an absorption maximum about 500 nm, which was also red-shifted by 25 nm from the ADS12PQT polymer solution. Since the ADS12PQT has longer conjugation than the PURET, the ADS12PQT nanoparticles have larger wavelength shift in its absorption maximum in comparison to that for PURET nanoparticles. When excited at their respective absorption peak wavelengths, strong fluorescence from the PURET and ADS12PQT nanoparticles were observed with their emission maxima around 625 nm and 675 nm, respectively. The ADS12PQT nanoparticles showed two peaks in its emission spectrum.
The particle sizes of the polymer nanoparticles were determined by dynamic light scattering (DLS) and confirmed by scanning electron microscopy (SEM). The average size obtained for the PURET nanoparticles was 90 nm. Fig. 3(A) shows the SEM image of the PURET nanoparticles. The particles size distribution for the PURET nanoparticles as obtained from DLS is shown in Fig. 3(B). The particle size distribution and the SEM image of the ADS12PQT nanoparticles are provided in the ESI as Fig. S1 and S2,† respectively. TPF was measured from these nanoparticles dispersions with the near-IR laser excitation at 800 nm from a mode-locked Ti:sapphire laser. The TPF observed from the PURET and ADS12PQT nanoparticles demonstrated that the nanoparticles formed still possess the nonlinear optical property of the polymers. When the power of the incident laser beam was increased, simultaneous increase in the fluorescence intensity was observed. Fig. 4(A) and (B) show the intensity dependent behaviour of the TPF from the PURET and ADS12PQT nanoparticles, respectively. There are slight spectral mismatch between the one-photon fluorescence and the TPF. The mismatch could be mainly attributed to the difference in the spectral responses of the two detection systems used. In addition, the difference in the exciting wavelengths in the one-photon emission and TPF might alter the shapes of the fluorescence spectra as well. The laser power dependent TPF from the nanoparticles are shown in Fig. S3 in the ESI.† The least squares fitting of the log–log plots yielded the slopes close to 2, confirming that the fluorescence signals measured from the polymer nanoparticles dispersions were resulted from the TP processes.
 | ||
| Fig. 3 (A) SEM image of the PURET nanoparticles; and (B) the particle size distribution determined by DLS. | ||
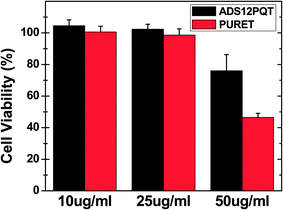
Unintended cytotoxicity of nanoparticles intended for bioimaging pose a serious limitation to their possible use in biomedical applications. Hence, the biocompatibility of any fluorescent material for any envisioned biological application is a very important attribute.18 In this study, the MTT cell viability assay was used to determine the effect of the PURET and ADS12PQT nanoparticles on proliferation of THP-1 cells. For MTT assay, the cells were seeded in 200 μL of complete culture medium containing 10–50 μg mL−1 mass concentrations of the PURET and ADS12PQT nanoparticles in 96-well plates for 24 h incubation time. There was no evidence of any morphology change when the cells were viewed under a phase-contrast microscope indicating that the TP active nanoparticles have little or no cytotoxicity.18 As shown in Fig. 5, treatment with the PURET and ADS12PQT nanoparticles (10–50 μg mL−1) did not affect the viability of the THP-1 cells, but some dose dependent inhibition was seen for both nanoparticles at the highest tested concentration. However, it has to be pointed out, due to strong fluorescence signal generally obtained from conjugated polymer, the required polymer concentrations for potential bioimaging applications would be lower in comparison to one photon active dyes and molecules.18In vitro TP imaging for the uptake of TP active nanoparticles was performed using a Zeiss laser scanning (at 800 nm) confocal and multi-photon system. Fig. 6 depicts the uptakes of both the PURET and ADS12PQT nanoparticles by the macrophages after 24 h incubation with 50 μg mL−1 nanoparticle dose. Moreover, TPF microscopy also confirmed the TP activity of the engulfed nanoparticles. The cellular internalization of the TP active nanoparticles were further investigated by Z-stacking (Fig. S4 and S5†). For Z-stacking, images were collected at 0.7 μm intervals to create a stack in the Z-axis.
In summary, TP active nanoparticles of two conjugated polymers were fabricated using a facile miniemulsion technique. The nanoparticles were characterized by DLS and SEM. Strong TPF was observed from the aqueous dispersion of the polymer nanoparticles and the quadratic dependence of the TPF on the laser intensity was confirmed. The luminescent polymeric nanoparticles were found to be non-toxic and microscopic studies confirmed internalization of TP active nanoparticles by the macrophages. Thus, these fluorescent nanoparticles could be suitable for high contrast in vitro TP bioimaging. Polymer based nanoparticles have extensively been used in nanomedicine, with encapsulation or conjugation of active drugs and fluorescent molecules. The TP active polymer nanoparticles studied here can be further utilized in diverse in vitro/in vivo bioimaging applications. Moreover, TP active polymer encapsulated nanoparticles hold tremendous potential for non-specific or targeted cellular imaging and drug delivery applications.
Acknowledgements
Financial support from the US Department of Energy is gratefully acknowledged. This research was also supported in part by an appointment to the Faculty Research Participation Program at the US Army Natick Soldier Research, Development and Engineering Centre (NSRDEC) administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and NSRDEC. SS would also like to thank the Tripathy Summer Graduate Fellowship from the University of Massachusetts Lowell. AKP would also like to thank the Graduate Research Grant Award 2011–2012 from the Graduate Student Association at University of Massachusetts Lowell. We are also grateful to Dr Suresh Gadde at the Brigham and Women's Hospital for guidance and suggestions with the research and manuscript.References
- I. J. Cox, C. J. R. Sheppard and T. Wilson, Appl. Opt., 1982, 60, 391 Search PubMed.
- M. Le Puil, J. P. Biggerstaff, B. L. Weidow, J. R. Price, S. A. Naser, D. C. White and R. S. Alberte, J. Microbiol. Methods, 2006, 67, 597 CrossRef CAS PubMed; A. Miyawak, Microscopy, 2013, 62, 63 CrossRef PubMed.
- W. Denk, J. H. Strickler and W. W. Webb, Science, 1990, 248, 73 Search PubMed; Z. Yu, J. Am. Chem. Soc., 2013, 135(8), 2883 CrossRef CAS PubMed.
- J. Chen, I. R. Corbin, H. Li, W. Cao, J. D. Glickson and G. Zheng, J. Am. Chem. Soc., 2007, 129, 5798 CrossRef CAS PubMed.
- R. R. Birge, Acc. Chem. Res., 1986, 19, 138 CrossRef CAS.
- E. B. Brown, R. B. Campell, Y. Tsuzuki, L. Xu, P. Carmeliet, D. Fukumura and R. Jain, Nat. Med., 2001, 7, 864 CrossRef CAS PubMed.
- K. Gaus, E. Gratton, E. P. W. Kable, A. S. Jones, I. Gelissen, L. Kritharides and W. Jessup, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 15554 CrossRef CAS PubMed.
- L. Fu, A. Jain, C. Cranfield, H. Xie and M. Gu, J. Biomed. Opt., 2007, 12, 040501 CrossRef PubMed.
- N.-N. Dong, M. Pedroni, F. Piccinelli, G. Conti, A. Sbarbati, J. E. R. Hernández, L. M. Maestro, M. C. I. de la Cruz, F. S. Rodriguez, A. Juarranz, F. Chen, F. Vetrone, J. A. Capobianco, J. GarcíaSolé, M. Bettinelli, D. Jaque and A. Speghini, ACS Nano, 2011, 5, 8665 CrossRef CAS PubMed.
- V. Lebret, L. Raehm, J.-O. Durand, M. Smaïhi, M. H. V. Werts, M. Blanchard-Desce, D. MéthyGonnod and C. Dubernet, J. Biomed. Nanotechnol., 2010, 6, 176 CrossRef CAS PubMed.
- M. A. Albota, C. Xu and W. W. Webb, Appl. Opt., 1998, 37, 7352 CrossRef CAS.
- W. Jung, S. Tang, D. T. McCormic, T. Xie, Y.-C. Ahn, J. Su, I. V. Tomov, T. B. Krasieva, B. J. Tromberg and Z. Chen, Opt. Lett., 2008, 33, 1324 CrossRef.
- C. Kontogiorgis, A. Detsi and D. Hadjipavlou-Litina, Expert Opin. Ther. Pat., 2012, 22, 437 CrossRef CAS PubMed; M. E. Riveiro, N. De Kimpe, A. Moglioni, R. Vazquez, F. Monczor, C. Shayo and C. Davio, Curr. Med. Chem., 2010, 17, 1325 CrossRef.
- M. Albota, D. Beljonne, J. L. Brédas, J. E. Ehrlich, J. Y. Fu, A. A. Heikal, S. E. Hess, T. Kogej, M. D. Levin, S. R. Marder, D. McCord-Maughon, J. W. Perry, H. Röckel, M. Rumi, G. Subramaniam, W. W. Webb, X. L. Wu and C. Xu, Science, 1998, 281, 1653 CrossRef CAS.
- E. V. Keuren, T. Wakebe, R. Andreaus, H. Mohwald, W. Schrof, V. Belov, H. Matsuda and R. R. Rojo, Appl. Phys. Lett., 1999, 75, 3312 CrossRef.
- S. Satapathi, L. Li, A. Kumar, H. Huo, R. Anandakathir, M. Shen, L. Samuelson and J. Kumar, Opt. Commun., 2011, 4, 3612 CrossRef PubMed.
- K. G. Chittibabu, S. Balasubramanian, W. H. Kim, A. L. Cholli, J. Kumar and S. K. Tripathy, J. Macromol. Sci., Part A: Pure Appl.Chem., 1996, 33, 1283 CrossRef.
- K. Li and B. Liu, J. Mater. Chem., 2012, 22, 1257–1264 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra44576c |
| ‡ Equal authors. |
| This journal is © The Royal Society of Chemistry 2014 |