An operationally simple, palladium catalysed dehydrogenative cross-coupling reaction of pyridine N-oxides and thiazoles “on water”†
Nicky J. Willisa and
James M. Smith*b
aSchool of Biological and Chemical Science, Queen Mary University of London, Mile End Road, London, E1 4NS, UK. E-mail: n.j.willis@qmul.ac.uk; Fax: +44 (0)20-7882-7427; Tel: +44 (0)20-7882-3271
bCancer Research Technology Ltd., Discovery Laboratories, Jonas Webb Building, Babraham Research Campus, Cambridge, CB22 3AT, UK. E-mail: jsmith@cancertechnology.com; Tel: +44 (0)12-2349-4008
First published on 12th February 2014
Abstract
Herein we describe the first reported example of dehydrogenative cross-coupling of heteroaromatic moieties achieved “on water”. This reaction is catalytic in palladium, site selective and operationally simple.
Due to the well documented importance of C2-heteroarenes, in the fields of pharmaceuticals, materials and natural products, there has been a large volume of work carried out towards the efficient construction of these valuable molecular scaffolds.1 These procedures usually require pre-functionalised starting materials such as organometallics and/or aryl-halides to facilitate site-specific transition metal catalysed cross-coupling reactions.2 However in recent years there have been an increasing number of pioneering reports describing dehydrogenative cross-coupling reactions that are capable of generating these important motifs.3 Perhaps the most significant barriers to progress are achieving regioselective control and preventing competitive homo-coupling pathways.
A further drawback for these procedures has been the frequent requirement for high temperatures and therefore high boiling solvents, such as DMF or DMA. In recent years there have been an increasing number of reports that C–H activation of arenes can be achieved “on water”.4 Once activated these intermediates subsequently undergo transition metal catalysed cross-coupling reactions with aryl halides.5
To advance this methodology further, we anticipated that a protocol of this type could perhaps be extended to non-halogenated heterocycles. This would provide a proof of concept towards the ultimate ambition of high yielding, site-selective oxidative hetero-cross coupling reactions “on water”.6
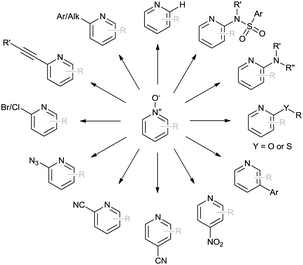
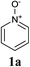
When considering inherent electronic bias of heterocycles, due to recent advances in pyridine N-oxide chemistry, these moieties are both ideal candidates for C–H activation and highly-valuable, synthetically-flexible pyridine building blocks (Scheme 1).7 Increasingly, synthetic N-oxide modification can be performed by in situ enhancement of the inherent electrophilic nature of the 2-position via co-ordination of an appropriate activating agent.
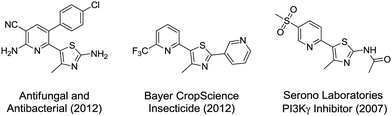
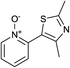
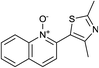
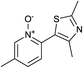
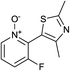
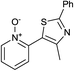
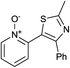
In terms of a complementary cross-coupling partner, due to the appropriately activated electron-rich aromatic character of thiazoles, these versatile substrates were similarly considered suitable for such C–H activation studies.3k Furthermore, with a view to demonstrate the application of such chemistry towards compounds of interest, the coupling of pyridine N-oxides with thiazoles would then allow potentially rapid access from the direct products to the important pyridyl-thiazole class of biologically active molecules8 (Fig. 1).
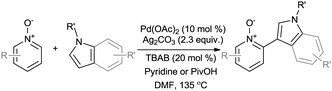
This also allowed us to build upon the work of Li and Zhang, who recently exploited the electronic bias of pyridine N-oxides to undergo dehydrogenative cross-coupling with indoles (Scheme 2).3k This procedure facilitates oxidative cross-coupling in generally good yields (45–81%), however requires the use of DMF as a solvent, which has environmental and practical limitations in terms of toxicity, high boiling temperature and miscibility with water.
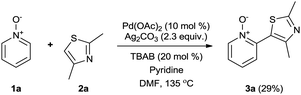
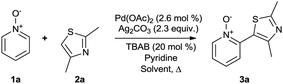
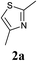
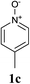
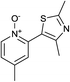
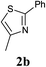
Our initial synthetic efforts focused upon employing 2,3-dimethyl thiazole as a simple chemical probe (due to the simplification of the regiochemical outcomes) in a dehydrogenative cross coupling reaction with pyridine N-oxide (Scheme 3). In our preliminary studies, it was gratifying to observe conversion to the desired product using Li's experimental procedure, however in a somewhat modest yield (29%).
The reaction was then screened against a variety solvents at 80 °C for direct comparison. It was with great delight that we observed that this reaction could be achieved in a comparable yield (28%, entry 5, Table 1), “on water” in our initial experiment despite a 55 °C reduction in temperature. Upon increasing the temperature to 100 °C, it was found that this conversion could again be slightly improved (entry 6, Table 1).
| Entry | Solvent | Temp. °C | Yield % |
|---|---|---|---|
| a Reaction carried out according to procedure II (see ESI). | |||
| 1 | DMF | 135 | 28 |
| 2 | THF | 80 | 19 |
| 3 | MeCN | 80 | 0 |
| 4 | PhMe | 80 | 8 |
| 5 | H2O | 80 | 28 |
| 6 | H2O | 100 | 33 |
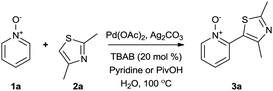
After further screening it was found that conducting the reaction under a N2 atmosphere had no affect on product formation and that the reaction could be carried out in an atmosphere of air, making the procedure operationally facile (entry 1, Table 2). Interestingly, it was found that a TBAB was imperative to the reaction (entries 3 and 4, Table 2), which may support the theory of Li and Zhang that it has an important role in stabilising the Ag2CO3 or another relevant silver species. However in our “on-water” solvent-system it may also be acting as a phase transfer catalyst, as it was later noted that TBAF could also be used to achieve this transformation (entry 21, Table 2).
| Entry | Pd (mol%) | Pyridine (equiv.) | P.N.O. (equiv.) | Ag2CO3 (equiv.) | Yielda (%) |
|---|---|---|---|---|---|
| a Conversion calculated by 1H NMR relative to an internal standard.b Condition: TBAB replaced with PivOH (2.3 equiv.) as an additive.c Condition: reaction carried out in the absence of TBAB.d Condition: reaction was carried out at 0.375 M concentration of thiazole.e Condition: reaction was carried out at 0.75 M concentration of thiazole.f Condition: reaction was carried out in the presence of X-Phos (10 mol%) as ligand.g Condition: reaction was carried out at 0.75 M concentration of thiazole in the presence of X-Phos (10 mol%) as ligand.h Condition: X-Phos replaced with PPh3.i Condition: X-Phos replaced with Xantphos.j Condition: X-Phos replaced with BINAP.k Condition: TBAB replaced with TBAF.l Condition: Ag2CO3 replaced with K2CO3.m Condition: Ag2CO3 replaced with AgOAc. | |||||
| 1 | 2.6 | 4.0 | 4.0 | 2.3 | 34 |
| 2 | 2.6 | 0.0 | 4.0 | 2.3 | 8 |
| 3 | 2.6 | 4.0 | 4.0 | 2.3 | 0b |
| 4 | 2.6 | 4.0 | 4.0 | 2.3 | 1c |
| 5 | 5.2 | 4.0 | 4.0 | 2.3 | 36 |
| 6 | 7.8 | 4.0 | 4.0 | 2.3 | 37 |
| 7 | 10.4 | 4.0 | 4.0 | 2.3 | 37 |
| 8 | 2.6 | 4.0 | 4.0 | 2.3 | 52d |
| 9 | 2.6 | 4.0 | 4.0 | 2.3 | 66e |
| 10 | 2.6 | 8.0 | 4.0 | 2.3 | 40 |
| 11 | 2.6 | 6.0 | 4.0 | 2.3 | 34 |
| 12 | 2.6 | 4.0 | 4.0 | 3.0 | 47 |
| 13 | 2.6 | 4.0 | 6.0 | 2.3 | 55 |
| 14 | 2.6 | 4.0 | 8.0 | 2.3 | 53 |
| 15 | 2.6 | 4.0 | 4.0 | 2.3 | 45f |
| 16 | 2.6 | 4.0 | 6.0 | 3.0 | 80g |
| 17 | 2.6 | 4.0 | 6.0 | 3.0 | 78g,h |
| 18 | 2.6 | 4.0 | 6.0 | 3.0 | 76g,i |
| 19 | 2.6 | 4.0 | 6.0 | 3.0 | 60g,j |
| 20 | 2.6 | 4.0 | 3.0 | 3.0 | 68g |
| 21 | 2.6 | 4.0 | 6.0 | 3.0 | 71g,k |
| 22 | 2.6 | 4.0 | 6.0 | 3.0 | 0g,l |
| 23 | 2.6 | 4.0 | 6.0 | 3.0 | 60g,m |
Variation of catalyst concentration also had a negligible effect on product formation, (entries 5–7, Table 2), indicating that palladium concentration is not rate limiting in this system.
Increasing the reaction concentration and the amount of pyridine-N-oxide did however lead to improved conversion, suggesting its involvement in the rate-determining step (entries 13 and 14, Table 2). Increasing the amount of Ag2CO3 and addition of X-Phos to stabilise the catalyst were also beneficial (entries 12 and 15, Table 2) and by combining these changes the conversion could be increased to 80% in 22 h (entry 16, Table 2). A number of phosphorus ligands were found to be useful to achieve this transformation, however no improvements with respect to overall yield were gained (entries 17–19, Table 2).
The role of silver as an oxidant, inferred from the silver mirroring on the reaction vessel was confirmed by the absence of any product formation upon replacing Ag2CO3 with K2CO3 (entry 22, Table 2). This transformation could however be achieved using the more soluble AgOAc as a base (entry 23, Table 2) but in markedly reduced yield (60%) presumably due to the reduction in basicity.
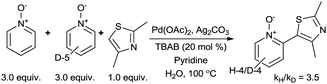
To confirm this role of the N-oxide in the rate determining step, Li and Zhang's intermolecular competition experiment between pyridine and pyridine-d5 N-oxide was replicated under our optimised reaction conditions (Scheme 4). This hypothesis was further supported by observation of a primary kinetic isotope effect (kH/kD = 3.5), which was highly comparable to that obtained by Li and Zhang (kH/kD = 3.6).
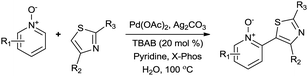
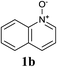
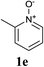
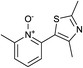
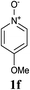
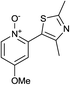
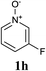
Upon probing the scope of the N-oxides, it was found that quinoline N-oxides can also be coupled using this procedure (entry 2, Table 3) in 64% isolated yield. The reaction also tolerates pyridine N-oxides functionalised with electron-donating groups (entries 3–6, Table 3) in good overall yields (58–72%) and excellent regioselectivity. It should however be noted that the methyl substituted pyridine N-oxides required increased palladium loading (from 2.6 to 10 mol%) and longer reaction times (from 22 to 48 h) to consume the starting material.
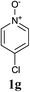
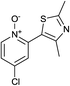
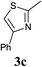
The reaction also tolerated halogenated substitution (entries 7 and 8, Table 3), however with more modest overall conversion even when using a higher catalyst loading. Gratifyingly thiazoles bearing aromatic moieties could also be employed without incident (entries 9 and 10, Table 3). This therefore opens up the scope of the reaction towards the rapid atom and step-efficient synthesis of biaryl-thiazoles analogues from these useful N-oxide building blocks.
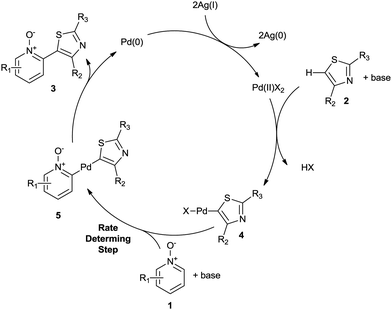
Although a full mechanistic pathway for this process remains to be elucidated, based on current experimental observations a reaction mechanism has been proposed (Scheme 5). It appears plausible that in the thiazole 2 undergoes C–H metalation to generate the heteroaryl-Pd(II) species 4.
This reactive species, then undergoes a second C–H metalation reaction with the N-oxide to give 5 before undergoing reductive elimination to generate the desired C2-heteroarene 3 and Pd(0), which is then re-oxidised by Ag(I) (2.0 equiv.).
Conclusions
In conclusion, we have to our knowledge demonstrated the first example of a heteroaromatic cross-coupling via C–H activation of both coupling partners in an aqueous media. This protocol is regioselective with respect to the N-oxide, operationally simple and doesn't require an inert atmosphere. It is anticipated that in subsequent investigations that the N-oxide component or molecular oxygen may be used as an internal oxidant, thus reducing the amount of Ag2CO3 required.9 It is also anticipated that substrate scope could be expanded to include other electron rich heterocycles.Acknowledgements
We wish to thank the EPSRC (EP/G041431/1) and Queen Mary ImpactQM scheme (EP/H500162/1) for funding, Cancer Research Technology Ltd. for hosting this placement and the EPSRC National Mass Spectrometry Service Centre, Swansea for obtaining HRMS data. Further thanks are owed to Dr Martin Stockley for practical advice, and Dr Martin E. Swarbrick, Dr Christopher D. Bray and Dr Tanya C. Boorman for their guidance in the preparation of this manuscript.Notes and references
-
(a) K. Okamato, J. Zhang, J. B. Housekeeper, S. R. Marder and C. K. Luscombe, Macromolecules, 2013, 46, 8059 CrossRef
; (b) V. P. Mehta and B. Punji, RSC Adv., 2013, 3, 11957 RSC
; (c) J. P. Michael, Nat. Prod. Rep., 2005, 22, 627 RSC
; (d) G. D. Henry, Tetrahedron, 2004, 60, 6043 CrossRef CAS
.
-
(a) D. Zhao, J. You and C. Hu, Chem.–Eur. J., 2011, 17, 5466 CrossRef CAS PubMed
; (b) X. Chen, K. M. Engle, D. Wang and J. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed
; (c) K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442 CrossRef CAS PubMed
.
-
(a) L. Zhao, C. Tsukano, E. Kwon, Y. Takemoto and M. Hirama, Angew. Chem., Int. Ed., 2013, 52, 1722 CrossRef CAS PubMed
; (b) B. Liu, Y. Huang, J. Lan, F. Song and J. You, Chem. Sci., 2013, 4, 2163 RSC
; (c) X. Qin, H. Liu, D. Qin, Q. Wu, J. You, D. Zhao, Q. Guo, X. Huang and J. Lan, Chem. Sci., 2013, 4, 1964 RSC
; (d) Z. Wang, F. Song, Y. Zhao, Y. Huang, L. Yang, D. Zhao, J. Lan and J. You, Chem.–Eur. J., 2012, 18, 16616 CrossRef CAS PubMed
; (e) X. Fu, Q. Xuan, L. Liu, D. Wang, Y. Chen and C. Li, Tetrahedron, 2012, 69, 4436 CrossRef
; (f) X. Qin, B. Feng, J. Dong, X. Li, Y. Xue, Y. Lan and J. You, J. Org. Chem., 2012, 77, 7677 CrossRef CAS PubMed
; (g) M. Nishino, K. Hirano, T. Satoh and M. Miura, Angew. Chem., Int. Ed., 2012, 51, 6993 CrossRef CAS PubMed
; (h) J. Dong, Y. Huang, X. Qin, Y. Cheng, J. Hao, D. Wan, W. Li, X. Liu and J. You, Chem.–Eur. J., 2012, 18, 6158 CrossRef CAS PubMed
; (i) Z. Wang, K. Li, D. Zhao, J. Lan and J. You, Angew. Chem., Int. Ed., 2011, 50, 5365 CrossRef CAS PubMed
; (j) W. Han, P. Mayer and A. R. Ofial, Angew. Chem., Int. Ed., 2011, 50, 2178 CrossRef CAS PubMed
; (k) X. Gong, G. Song, H. Zhang and X. Li, Org. Lett., 2011, 13, 1766 CrossRef CAS PubMed
; (l) A. D. Yamaguchi, D. Mandal, J. Yamaguchi and K. Itami, Chem. Lett., 2011, 40, 555 CrossRef CAS
; (m) H. Do and O. Daugulis, J. Am. Chem. Soc., 2011, 133, 13577 CrossRef CAS PubMed
; (n) P. Xi, F. Yang, S. Qin, D. Zhao, J. Lan, G. Gao, C. Hu and J. You, J. Am. Chem. Soc., 2010, 132, 1822 CrossRef CAS PubMed
.
- S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275 CrossRef CAS PubMed
.
-
(a) P. B. Arockiam, C. Fischmeister, C. Bruneau and P. H. Dixneuf, Green Chem., 2013, 15, 67 RSC
; (b) K. S. Singh and P. H. Dixneuf, ChemCatChem, 2013, 5, 5 Search PubMed
; (c) Y. Su, Y. Deng, T. Ma, Y. Li and L. Sun, Green Chem., 2012, 14, 1979 RSC
; (d) F. Chen, Q. Min and X. Zhang, J. Org. Chem., 2012, 77, 2992 CrossRef CAS PubMed
; (e) L. Ackermann, J. Pospech and H. K. Potukuchi, Org. Lett., 2012, 14, 2146 CrossRef CAS PubMed
; (f) L. Joucla, N. Batail and L. Djakovitch, Adv. Synth. Catal., 2010, 352, 17 Search PubMed
; (g) T. Nishikata, A. R. Abela and B. H. Lipshutz, Angew. Chem., Int. Ed., 2010, 49, 781 CrossRef CAS PubMed
; (h) S. A. Ohnmacht, A. J. Culshaw and M. F. Greaney, Org. Lett., 2010, 12, 224 CrossRef CAS PubMed
; (i) D. K. Barange, V. Kavala, B. Rama Raju, C. Kuo, C. Tseng, Y. Tu and C. Yao, Tetrahedron Lett., 2009, 50, 5116 CrossRef CAS
; (j) S. A. Ohnmacht, P. Mamone, A. J. Culshaw and M. F. Greaney, Chem. Commun., 2008, 1241 RSC
; (k) G. L. Turner, J. A. Morris and M. F. Greaney, Angew. Chem., Int. Ed., 2007, 46, 7996 CrossRef CAS PubMed
.
- For recent reviews in dual C–H activation see
(a) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960–9009 CrossRef CAS PubMed
; (b) S. H. Cho, J. Y. Kim and S. Chang, Chem. Soc. Rev., 2011, 40, 5068–5083 RSC
; (c) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 3, 1215–1292 CrossRef PubMed
.
- Examples of reduction
(a) H. P. Kokatla, P. F. Thomson, S. Bae, V. R. Doddi and M. K. Lakshman, J. Org. Chem., 2011, 76, 7842 CrossRef CAS PubMed
; (b) S. H. Cho, S. J. Hwang and S. Chang, J. Am. Chem. Soc., 2008, 130, 9254 CrossRef CAS PubMed
; sulfonamide synthesis; (c) R. P. Farrell, M. Victoria, S. Elipe, M. D. Bartberger, J. S. Tedrow and F. Vounatsos, Org. Lett., 2013, 15, 168 CrossRef CAS PubMed
; (d) J. M. Keith, J. Org. Chem., 2012, 77, 11313 CrossRef CAS PubMed
; (e) W. J. Lominac, M. L. D'Angelo, M. D. Smith, D. A. Ollison and J. M. Hanna, Tetrahedron Lett., 2012, 53, 906 CrossRef CAS PubMed
; (f) A. T. Londregan, S. Jennings and L. Wei, Org. Lett., 2011, 13, 1840 CrossRef CAS PubMed
; amine and amide synthesis see ref. 3c and f; (g) A. T. Londregan, S. Jennings and L. Wei, Org. Lett., 2010, 12, 5254 CrossRef CAS PubMed
; (h) J. W. Medley and M. Movassaghi, J. Org. Chem., 2009, 74, 1341 CrossRef CAS PubMed
; (i) J. Yin, B. Xiang, M. A. Huffman, C. E. Raab and I. W. Davies, J. Org. Chem., 2007, 72, 4554 CrossRef CAS PubMed
; ether synthesis see ref. 3f; (j) A. T. Londregan, K. A. Farley, C. Limberakis, P. B. Mullins and D. W. Piotrowski, Org. Lett., 2012, 14, 2890 CrossRef CAS PubMed
; meta-arylation; (k) B. S. Shaibu, R. K. Kawade and R. Liu, Org. Biomol. Chem., 2012, 10, 6834 RSC
; (l) C. Raminelli, Z. Liu and R. C. Larock, J. Org. Chem., 2006, 71, 4689 CrossRef CAS PubMed
; nitrile synthesis; (m) E. L. Meredith, O. Ardayfio, K. Beattie, M. R. Dobler, I. Enyedy, C. Gaul, V. Hosagrahara, C. Jewell, K. Koch, W. Lee, H. Lehmann, T. A. McKinsey, K. Miranda, N. Pagratis, M. Pancost, A. Patnaik, D. Phan, C. Plato, M. Qian, V. Rajaraman, C. Rao, O. Rozhitskaya, T. Ruppen, J. Shi, S. J. Siska, C. Springer, M. van Eis, R. B. Vega, A. von Matt, L. Yang, T. Yoon, J. Zhang, N. Zhu and L. G. Monovich, J. Med. Chem., 2010, 53, 5400 CrossRef CAS PubMed
; (n) Z. Huo, T. Kosugi and Y. Yamamoto, Tetrahedron Lett., 2008, 49, 4369 CrossRef CAS
; (o) E. Ochiai, J. Org. Chem., 1953, 18, 534 CrossRef CAS
; azide synthesis; (p) D. M. Andrews, T. C. M. Page, J. M. Peach and A. J. Pratt, J. Chem. Soc., Perkin Trans. 1, 1995, 8, 1045 RSC
; halogenation; (q) S. E. Wengryniuk, A. Weickgenannt, C. Reiher, N. A. Strotman, K. Chen, M. D. Eastgate and P. S. Baran, Org. Lett., 2013, 15, 792 CrossRef CAS PubMed
; (r) S. J. Connon and A. F. Hegarty, Tetrahedron Lett., 2001, 42, 735 CrossRef CAS
; ortho-alkylation; (s) B. Yao, R. Song, Y. Liu, Y. Xie, J. Li, M. Wang, R. Tang, X. Zhang and C. Deng, Adv. Synth. Catal., 2012, 345, 1890 CrossRef
; (t) K. C. Nicolaou, A. E. Koumbis, S. A. Snyder and K. B. Simonsen, Angew. Chem., Int. Ed., 2000, 39, 2529 CrossRef CAS
; (u) G. A. Russell, L. Wang and C. Yao, J. Org. Chem., 1995, 60, 5390 CrossRef CAS
; ortho-arylation; (v) L. Campeau, D. R. Stuart, J. Leclerc, M. Bertrand-Laperle, E. Villemure, H. Y. Sun, S. Lasserre, N. Guimond, M. Lecavallier and K. Fagnou, J. Am. Chem. Soc., 2009, 131, 3291 CrossRef CAS PubMed
; (w) J. M. Keith, J. Org. Chem., 2008, 73, 327 CrossRef CAS PubMed
.
-
(a) M. Venkatesan, Nat. Prod. Res., 2012, 26, 223 CrossRef PubMed
; (b) T. Bretschneider, E. Franken, U. Görgens, M. Füßlein, A. Hense, J. Kluth, H. Schwarz, A. Köhler, O. Malsam, A. Voerste, A. Becker, US Pat., US2011212949A1, 2011
; (c) A. Quattropani, V. Pomel, T. Rueckle, T. Grippi-Vallotton, EU Pat., WO2007/082956A1, 2007
.
- For a recent review for strategies to overcome the “oxidant problem” see A. N. Campbell and S. S. Stahl, Acc. Chem. Res., 2012, 45, 851–863 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra44411b |
| This journal is © The Royal Society of Chemistry 2014 |